References
The introduction of inclisiran
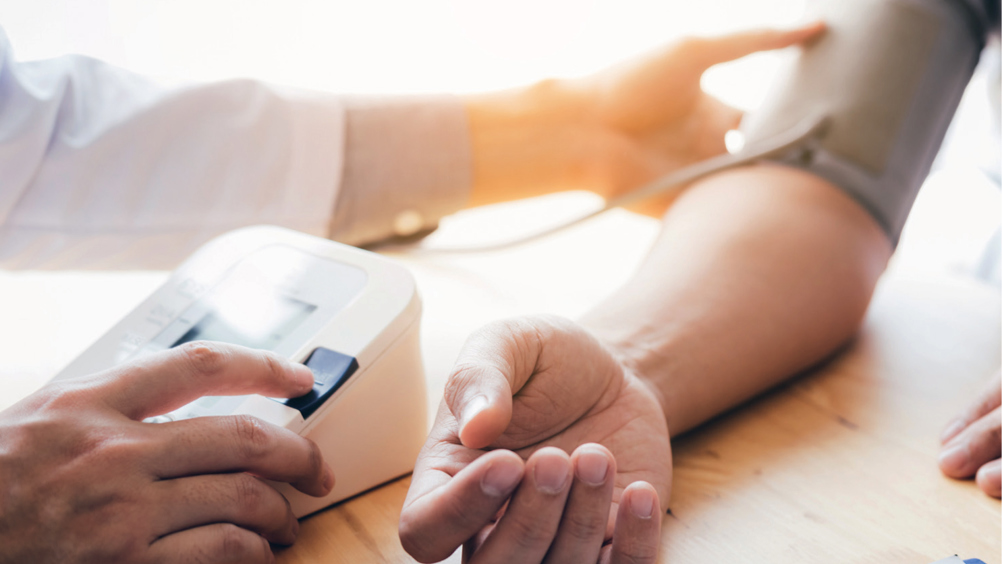
In January 2020, the UK Government's Department of Health and Social Care (DHSC) announced ‘a ground-breaking, in-principle agreement with Novartis’, namely, the introduction of inclisiran, (DHSC, 2020). Inclisiran interferes with RNA ‘to prevent synthesis of the enzyme PCSK9 (proprotein convertase subtilisin/kexin type 9), leading to lower serum concentrations of low-density lipoprotein cholesterol (LDL-C)’ (Byrne et al, 2020).
Byrne et al (2020) note that the NHS-Novartis agreement announced access to an unapproved drug at the start of the drug's clinical trial, and that ‘the government press release included no contact details for further information. Despite this, large clinical benefits are being claimed, even before the NHS trial has begun.’
On 9 December 2020 the European Medicines Agency (2020) approved inclisiran's use throughout the European Union (of which the UK is not a member). In January 2021 it was observed that Novartis would be attempting ‘to recoup some of the $9.7 billion it spent in acquiring the drug's developer, The Medicines Company’ (Anonymous, 2021); and in September 2021 it was reported that the National Institute for Health and Care Excellence (NICE) had approved ‘Leqvio® (inclisiran) following a Phase III trial that showed that the drug provided effective and sustained LDL-C reduction in two sub-populations of atherosclerotic cardiovascular disease: established cerebrovascular disease and polyvascular disease’ (Begley, 2021). Endorsing inclisiran as a ‘potential game-changer in preventing thousands of people from dying prematurely from heart attacks and strokes,’ NICE's deputy chief executive Meindert Boysen was clear that this ‘could see as many as 300 000 people with high cholesterol or mixed dyslipidaemia who have already had a previous cardiovascular event receive the drug over the next three years’ (Begley, 2021).
Register now to continue reading
Thank you for visiting Journal of Prescribing Practice and reading some of our peer-reviewed resources for prescribing professionals. To read more, please register today. You’ll enjoy the following great benefits:
What's included
-
Limited access to our clinical or professional articles
-
New content and clinical newsletter updates each month

