Numerous conditions, including cystic fibrosis, some cancers and autoimmune diseases, and nasal septum deviation, can cause rhinitis (inflammation of the nasal mucosa) (Scadding et al, 2008), which is characterised by one or more of four symptoms: nasal congestion, rhinorrhoea (‘runny nose’), sneezing, and itching (Dykewicz et al, 2020; Scadding et al, 2017). However, Allergic rhinitis (AR) accounts for about half of rhinitis cases (Bousquet et al, 2020) and is the most common chronic allergic disease in Europe (Bjermer et al, 2019). In some countries, up to half the population has AR (Bousquet et al, 2020).
Rhinitis can arise from several allergic and nonallergic mechanisms (eg infectious and drug-mediated rhinitis) (Bousquet et al, 2020; Dykewicz et al, 2020). For instance, allergic responses to vegetable and animal proteins and certain chemicals as well as non-allergic mechanisms (eg isocyanates, persulfate salts and woods) can cause occupational rhinitis (Bousquet et al, 2020).
In the UK, allergens (immune triggers) in the faeces of house dust mites, pollens from trees and grasses, spores from moulds and other fungi and the dander and saliva from a menagerie of animals (including cats, dogs, horses, cattle, rabbits and rodents) are the most common causes of AR (NHS, 2019).
Despite being common, AR management can present challenges (Bjermer et al, 2019). Indeed, between 30% and 60% of people with AR (PwAR) report that conventional treatments, such as antihistamines and intranasal steroids (INS), do not adequately reduce symptoms (Drazdauskaitė et al, 2020).
This review introduces the pharmacology of the main drugs used in primary care to treat AR. Additional considerations influence treatment choice in some groups of PwAR including the elderly, children, pregnant women and those with concurrent asthma (Bousquet et al, 2020). This feature does not consider these issues. Healthcare professionals should check the British National Formulary and Summary of Product Characteristics for each drug as well as guidelines, such as those published by the British Society for Allergy & Clinical Immunology (BSACI) (Scadding et al, 2017), for full details of diagnosis and management.
The impact of AR
Many PwAR experience mild symptoms (Dykewicz et al, 2020) and dismiss AR as little more than a nuisance (Bjermer et al, 2019). Nevertheless, between 35% and 50% of adults with AR report that nasal allergies have at least a moderate effect on their daily life (Dykewicz et al, 2020), such as impairing their social activities, sleep quality, cognition and mood, increasing their risk of anxiety, depression and learning difficulties, and undermining their work and school performance (Bjermer et al, 2019; Drazdauskaitė et al, 2020; Meng et al, 2020). Indeed, AR may undermine work productivity more than any other chronic disease, exceeding the impact of heart disease and diabetes combined (Bjermer et al, 2019).
In addition, PwAR often develop symptoms in the eye (rhinoconjunctivitis), sinuses (rhinosinusitis), middle ear, nasopharynx and lower airways (Scadding et al, 2017). Ocular symptoms and asthenia (physical weakness and a lack of energy) associated with AR can have an even greater effect on quality of life than nasal obstruction and pruritus (Bjermer et al, 2019).
The unified airway theory
Many PwAR have atopic dermatitis (Bousquet et al, 2020). AR also increases the risk of developing asthma (Scadding and Walker, 2012; Scadding et al, 2017). AR, atopic dermatitis and asthma share several disease-related genes, which partly accounts for the overlap (Bousquet et al, 2020). Moreover, the respiratory tract runs from the nasal vestibule to the alveoli (Kariyawasam and Rotiroti, 2013). Epidemiological, pathophysiological and clinical evidence, such as the association between AR and asthma discussed below, suggests that the upper and lower airways are a single functional and morphological unit (Haccuria et al, 2018; Niimi, 2021; Samitas et al, 2018). According to unified airway theory, AR, asthma, polyposis and some other diseases are on ‘a continuum of inflammation'within one airway (Grossman, 1997).
About 75% to 80% of people with asthma also have AR. The proportion rises to almost 100% of those with allergic asthma (Scadding and Walker, 2012; Dykewicz et al, 2020). People with concurrent AR typically experience more difficult-to-control or severe asthma than those with the latter alone (Dykewicz et al, 2020). Therefore, healthcare professionals should consider rhinitis, which may be allergic or non-allergic, in people with poorly controlled asthma (Scadding and Walker, 2012). Improved treatment of rhinitis should improve asthma control and healthcare professionals should treat both conditions, when present (Scadding and Walker, 2012).
Immunology
The immunology underling AR is fascinating and there is still much to learn (Meng et al, 2020). There is not the space to offer more than a broad outline. The informative reviews by Bousquet et al (2020), Meng et al (2020) and Drazdauskaitė et al (2020) offer comprehensive summaries.
Essentially, sensitisation occurs the first time that a PwAR encounters an allergen. However, sensitised PwAR do not, develop symptoms (Bousquet et al, 2020). During subsequent exposures, PwAR experience the early- and late-phase responses (Figure 1) that characterise AR (Dhruve, 2018; Bjermer et al, 2019).
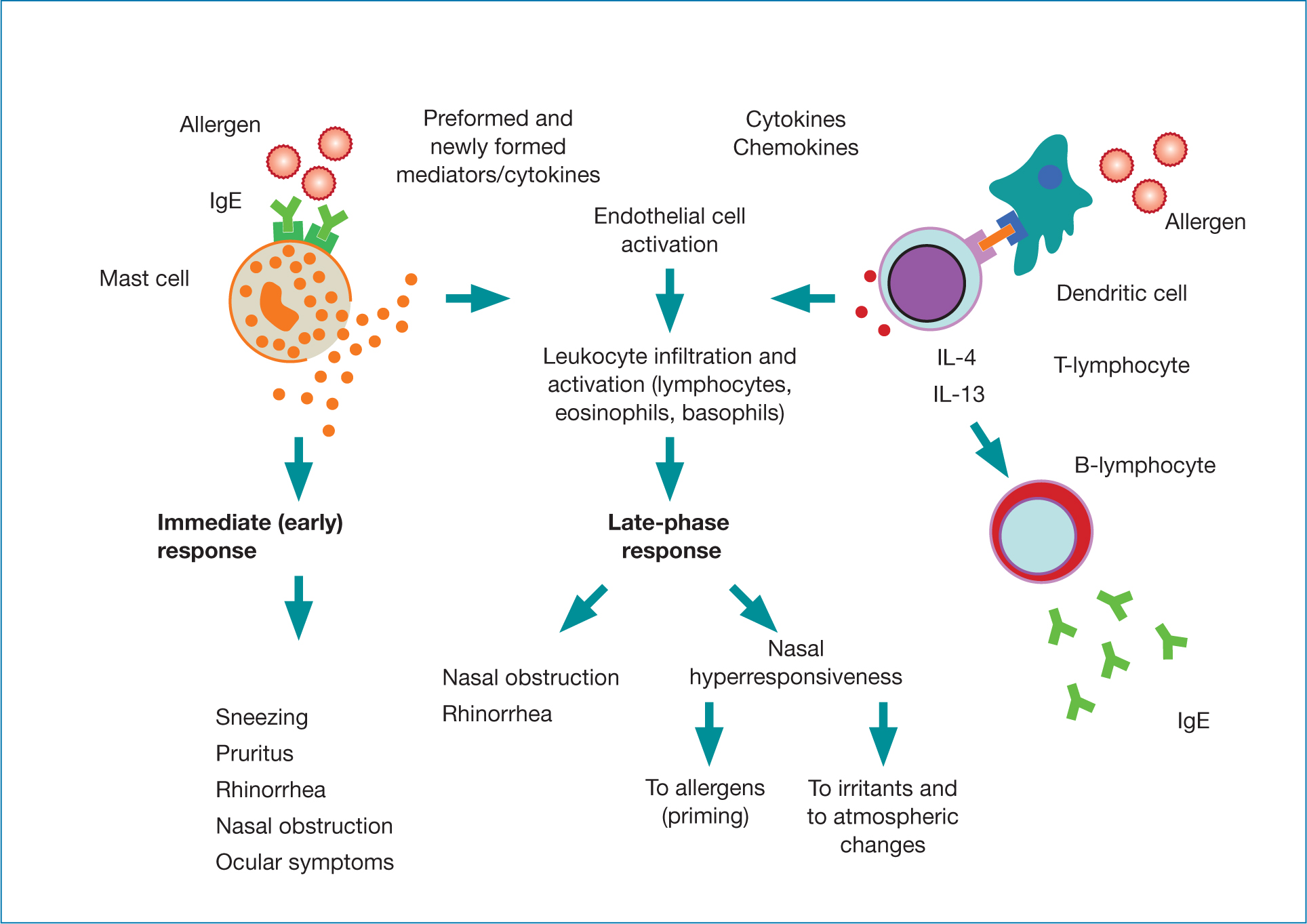
During the early-phase response, sensitised PwAR produce large amounts of immunoglobulin E (IgE) that is specific for the allergen (eg pollen from a particular species of grass or tree).
Specific receptors on mast cells and basophils recognise the IgE. Mast cells carry histamine in packets in their cytoplasm called granules. IgE's binding triggers mast cells, in PwAR particularly those in the nasal mucosa, to degranulate, releasing histamine. Over the next few minutes, PwAR develop acute nasal (eg sneezing) and ocular symptoms. Histamine, in conjunction with other pro-inflammatory mediators (eg leukotrienes) and eicosanoids (eg prostaglandins and kinins), increases the permeability of blood vessels, causing oedema (Bjermer et al, 2019). Several hours later, PwAR develop a ‘late-phase reaction’. White blood cells (basophils, neutrophils, T-lymphocytes, monocytes and eosinophils) release inflammatory mediators, such as cytokines, prostaglandins and leukotrienes. This perpetuates the nasal inflammation and can remodel tissue, exacerbate oedema and give rise to, and sustain, nasal congestion (Bjermer et al, 2019).
After repeated exposures to the allergen, the nose becomes primed. In other words, progressively smaller amounts of the allergen trigger AR symptoms. This may explain why PwAR develop symptoms to low pollen levels towards the end of the season (Bousquet et al, 2020). In addition, the nasal passages of PwAR can become hypersensitive. So, non-allergic factors, such as irritants, pollution, infections and environmental changes, also trigger and exacerbate AR symptoms (Bousquet et al, 2020).
PwAR can experience a spectrum of clinical symptoms that differ in frequency and severity. Some people experience intermittent AR, which the allergic rhinitis and its impact on asthma (ARIA) guidelines define as being present for fewer than 4 days a week or for fewer than 4 consecutive weeks. ARIA defines persistent symptoms as being present for more than 4 days a week and for more than 4 consecutive weeks (Bousquet et al, 2008).
The BSACI guidelines note that classification into seasonal and perennial rhinitis aids diagnosis and allergen-specific therapy (Scadding et al, 2017). Outdoor allergens (eg grass, weed and tree pollens) normally cause seasonal AR. Indoor allergens (eg dust mite, animal dander, some insects and mould) tend to cause perennial AR (Drazdauskaitė et al, 2020; Meng et al, 2020). However, most PwAR are sensitised to more than one allergen (polysensitised), which blurs the distinction between seasonal and perennial AR (Bjermer et al, 2019; Bousquet et al, 2020). Polysensitisation also tends to predict an earlier onset of, and more severe, allergic symptoms than sensitisation to a single allergen (Bousquet et al, 2020).
Pharmacological treatments
AR treatment should be individualised depending on symptoms, severity and duration (Bousquet et al, 2020). Many patients show poor symptom control despite self-medication (Bousquet et al, 2020). In addition, several drugs including nasal decongestants (see below), alpha-adrenergic antagonists, angiotensin converting enzyme inhibitors, chlorpromazine, cocaine, aspirin, nonsteroidal anti-inflammatory drugs, hormone replacement therapy and the contraceptive pill may trigger rhinitis (Scadding et al, 2008). Therefore, healthcare professionals should, therefore, ask which prescription and over-the-counter drugs the patient has tried for AR (Dykewicz et al, 2020). In addition, healthcare professionals may ask about diet: alcohol, spicy foods, pepper and sulphites can all trigger rhinitis in some people (Scadding et al, 2008).
Allergen avoidance is the foundation of AR management, but often proves difficult (Scadding et al, 2017). Healthcare professionals may, for example, suggest that PwAR use sunglasses, nasal filters, balms and ointments applied to the nose to reduce exposure to pollen (Scadding et al, 2017). In addition, healthcare professionals should advise PwAR patients to avoid irritants, including smoke and traffic pollution (Scadding et al, 2017). This is because the unified airway theory suggests smoke and traffic pollution also exacerbate and contribute to asthma (Bui et al, 2013; Künzli et al, 2009).
Adults and children with AR can also try nasal washout with saline, which is well tolerated and reduce the amount of pharmacotherapy PwAR need to control symptoms (Scadding et al, 2017).
Corticosteroids
Generally, specialists regard INS as the most effective drug for AR (Bjermer et al, 2019). For instance, INS are more effective in AR than oral H1-antihistamines or leukotriene receptor antagonists, particularly against nasal congestion (Bousquet et al, 2020). Some studies suggest that intranasal antihistamines (INAH) alleviate nasal and ocular symptoms as effectively as INS. Other studies report, however, that INS are more effective than INAH against nasal allergic symptoms (Dykewicz et al, 2020). Various INS are now available, with broadly similar efficacy in AR (Bjermer et al, 2019).
Short-courses of oral steroids (5–7 days) are more effective than INS, but at the risk of potentially serious systemic side-effects (see below), especially with high or repeated dosing (Dykewicz et al, 2020). Nevertheless, oral steroids may have a place as a short-term treatment for PwAR before an important event such as a wedding or exam (Scadding et al, 2008).
Mechanism of action
Corticosteroids produce broad anti-inflammatory effects. Natural glucocorticoids (a subclass of corticosteroid), such as hydrocortisone (also called cortisol) and cortisone, are anti-inflammatory. The adrenal cortex increases glucocorticoid production in response to inflammation (Ritter et al, 2020). The steroids used to treat AR evoke a stronger anti-inflammatory response than these endogenous corticosteroids.
Corticosteroids cross the cell membrane and bind to a specific receptor in the cytoplasm. Two of these complexes join together (dimerise) and move to the nucleus. Here, the dimer interacts with DNA ‘switching off’ some genes and ‘switching on’ others, such as inflammatory and anti-inflammatory genes respectively (Ritter et al, 2020). This takes time and healthcare professionals should remind PwAR that steroid anti-allergic benefits can take hours or days to emerge (Bousquet et al, 2020).
Steroid side effects
Glucocorticoids change the expression of about 1% of the human genome (Ritter et al 2020). So, glucocorticoids, especially at high doses or used for long periods, can cause side effects, including opportunistic infections, hyperglycaemia, central nervous system (CNS) adverse events, adrenal suppression, diabetes and osteoporosis (Dykewicz et al, 2020; Ritter et al, 2020). In general, INS are not absorbed sufficiently to cause clinically significant systemic adverse events at recommended doses. Nevertheless, the large variability in response suggests that prescribers should use the lowest effective dose for the shortest time and monitor children's growth (Bousquet et al, 2020; Dykewicz et al, 2020).
Despite the low risk of systemic adverse events, INS can cause side effects including dryness, burning, stinging, irritation, blood-tinged secretions, dryness and crusting, and epistaxis (Bousquet et al, 2020; Dykewicz et al, 2020; Ritter et al, 2020). A meta-analysis of 72 studies reported a 48% increased risk of epistaxis with INS compared with placebo in PwAR (Wu et al, 2019). Aiming the spray slightly away from the nasal septum seems to reduce the likelihood of developing these adverse events (Bousquet et al, 2020).
Antihistamines
Antihistamines bind strongly (with high affinity) to, and block, H1-receptors, which counters the increased vascular permeability and fluid leakage from capillaries that underlie rhinorrhoea, nasal pruritus and sneezing (Orzechowski et al, 2005; Bjermer et al, 2019).
Intranasal antihistamines work more rapidly than either INS or oral antihistamines. In PwAR, intranasal antihistamines typically show an onset of action in AR of between 15 and 30 minutes. This compares with an average of 150 minutes for oral antihistamines (Dykewicz et al, 2020). In people with seasonal AR, a nasal spray that combines azelastine and fluticasone propionate produces greater and more rapid improvement in symptoms, including rhinoconjunctivitis than either agent alone (Scadding et al, 2017).
Central effects
Broadly, first-generation H1-receptor antagonists (eg diphenhydramine, hydroxyzine, chlorpheniramine) cross the blood-brain barrier. Certain neurones in the CNS release histamine during the day. Levels fall at night. H1-receptors in the cortex and reticular activating system contribute to arousal and wakefulness (Ritter et al, 2020).
As a result, in some people first-generation antihistamines can impair psychomotor performance, including driving skills, even without the user feeling sedated (Orzechowski et al, 2005; Dykewicz et al, 2020). Therefore, certain antihistamines may increase the risk of traffic and occupational accidents, although the likelihood of developing sedative side-effects varies widely between users (Dykewicz et al, 2020).
In general, second-generation antihistamines (eg cetirizine, levocetirizine, fexofenadine, loratadine and desloratadine) do not cross the blood-brain barrier to the same extent as first-generation drugs and, therefore, reduce the risk of side-effects, such as sedation, impaired psychomotor performance and poor sleep quality (Dykewicz et al, 2020; Ritter et al, 2020). Nevertheless, some second-generation antihistamines still antagonise H1-receptors in the brain (Orzechowski et al, 2005).
Some antihistamines also antagonise muscarinic receptors or bind with other receptors (Orzechowski et al, 2005). Antihistamines differ in their affinity for muscarinic receptors. However, some antihistamines cause anticholinergic-mediated symptoms, such as dry eyes, dry mouth, constipation, urinary hesitancy and retention and tachycardia (Dykewicz et al, 2020, Orzechowski et al, 2005). Increasing evidence also suggests an association between the total exposure to anti-cholinergic actions (including side effects) and impaired cognitive and physical function in later life (Cebron Lipovec et al, 2020).
Intranasal antihistamines are generally well tolerated. Some people experience side effects such as bitter taste, headache, epistaxis and pharyngolaryngeal pain (Dykewicz et al, 2020). Between 15% and 20% of patients report that Intranasal antihistamines leave a bitter taste in the mouth (Bousquet et al, 2020).
Leukotriene receptor antagonists
Leukotriene receptor antagonists, such as montelukast, block a group of inflammatory mediators called cysteinyl leukotrienes that increase nasal congestion, mucus production and the number of inflammatory cells recruited to the nasal mucosa of PwAR (Bjermer et al, 2019). Leukotriene receptor antagonists have other anti-inflammatory actions, although further studies need to ascertain if these are relevant in PwAR at drug concentrations reached in the clinic (Bjermer et al, 2019). Leukotriene receptor antagonists show broadly similar efficacy to oral antihistamines (Bousquet et al, 2020).
As an aside, leukotrienes, cytokines and prostaglandins also seem to regulate sleep. This may partly account for the high rate of sleep disturbances among PwAR that are independent of symptoms such as nasal congestion (Bjermer et al, 2019).
‘[A] growing number of treatment options offer the opportunity to tailor treatment to each PwAR and help alleviate rhinitis's often marked impact on patients' lives’

Decongestants
Intranasal alpha-adrenergic agonists (eg oxymetazoline and xylometazoline) produce vasoconstriction and decrease nasal oedema. As a result, alpha-adrenergic agonists improve nasal conductance (‘unblock the nose’) for up to 10 hours. As alpha-adrenergic agonists do not reduce mediator release triggered by allergens, decongestants do not generally improve itching, sneezing or rhinorrhoea (Dykewicz et al, 2020).
Used continuously, alpha-adrenergic agonists can cause tachyphylaxis (the sensitivity of the tissue declines). This results in a worsening of rhinitis (rhinitis medicamentosa). Some people develop rhinitis medicamentosa within 3 days of daily use. Others do not develop rhinitis medicamentosa after 6 weeks of daily use. Intranasal decongestants can also cause nasal stinging or burning, sneezing, and dryness of the nose and throat (Dykewicz et al, 2020).
Immunotherapy
Currently, allergic specific immunotherapy (AIT) is the only treatment that resolves the immune changes that underly AR and, therefore, changes the disease trajectory (Meng et al, 2020). Patients receive AIT either subcutaneously or sublingually, although studies have explored other routes of administration (Drazdauskaitė et al, 2020; Meng et al, 2020). A Cochrane review concluded that sublingual immunotherapy ‘may be a safe option for people with well-controlled mild-to-moderate asthma and rhinitis who are likely to be at low risk of serious harm’ (Fortescue et al 2020).
To optimise and maintain the benefit, an AIT needs to last at least 3 years, either continuously or before the allergen season (Bousquet et al, 2020). Adherence to sublingual immunotherapy is initially between 44% and 97%. Nevertheless, fewer than 20% of patients progress to the third year of immunotherapy. The frequency of follow-up visits, perception of poor efficacy and cost contribute to poor adherence (Scadding et al, 2017). Patients who may require AIT must be referred to a hospital specialist for diagnosis, assessment and treatment (British National Formulary, 2022).
Conclusions
AR accounts for about half of rhinitis cases (Bousquet et al, 2020) and is the most common chronic allergy in Europe (Bjermer et al, 2019). AR management can present challenges (Bjermer et al, 2019) and many patients show poor symptom control despite self-medication (Bousquet et al, 2020). Nevertheless, a growing number of treatment options offer the opportunity to tailor treatment to each PwAR and help alleviate rhinitis's often marked impact on patients' lives.


