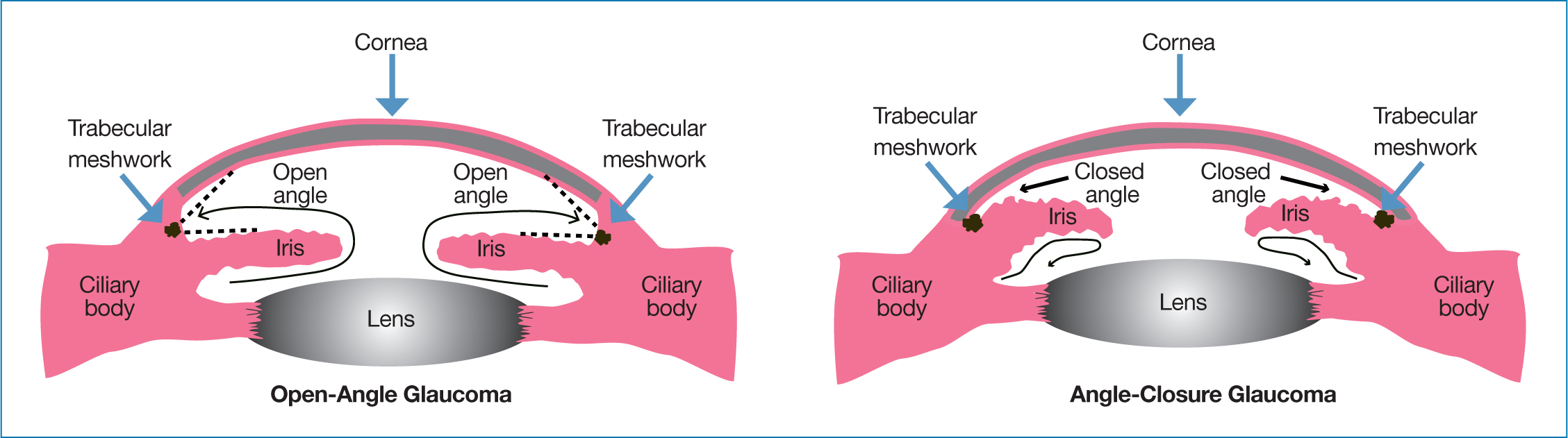
Glaucoma is the most common cause of irreversible visual loss worldwide (Sun et al, 2022). There are several types of glaucoma, ranging from primary congenital glaucoma, which occurs once in every 12 000 to 18 000 births, to chronic open-angle glaucoma (COAG), the most common variety, which affects about 480 000 people in England (European Glaucoma Society, 2017; Greener, 2019). COAG is about seven times more common than closed-angle (also called angle-closure) glaucoma, which is the next most frequent type (Gupta and Chen, 2016). Guidelines from the European Glaucoma Society (2017) provide full definitions of the various types.
Early diagnosis and rapid treatment are essential to preserve sight (Hamid et al, 2022). Intraocular pressure (IOP) is the only modifiable risk factor for glaucoma and reducing IOP is currently the only treatment strategy (Ho et al, 2017; Storgaard et al, 2021), although other targets are being explored that could result in new treatments (Greener, 2019). This article briefly introduces the pharmacological classes that reduce IOP and explores some factors that healthcare professionals (HCPs) should consider when using drugs to prevent sight loss, in particular emphasising the importance of concordance (adherence). The article also highlights the need for vigilance by all HCPs for side effects, such as iatrogenic glaucoma, which can arise from several commonly prescribed drugs (Table 1).
Table 1. Examples of drugs that can increase IOP
| Class | Examples |
|---|---|
| Adrenergic agonists | Phenylephrine (ophthalmic) |
| Salbutamol (inhaled) | |
| Anticholinergic drugs | Atropine (ophthalmic) |
| Botulinum toxin (intramuscular) | |
| Oxybutynin | |
| Scopolamine | |
| Antidepressants | Bupropion |
| Escitalopram | |
| Venlafaxine | |
| Anti-diabetes drugs | Sulphonylureas |
| Biguanides | |
| Cardiovascular drugs | Angiotensin converting enzyme inhibitors |
| Angiotensin receptor blockers | |
| Statins | |
| Steroids | Glucocorticoids (systemic and inhaled) |
| Sulphonamides | Acetazolamide |
| Methazolamide | |
| Topiramate | |
| Other drugs | Some cough mixtures, cough and flu remedies, certain anti-convulsant drugs |
Inside the eye
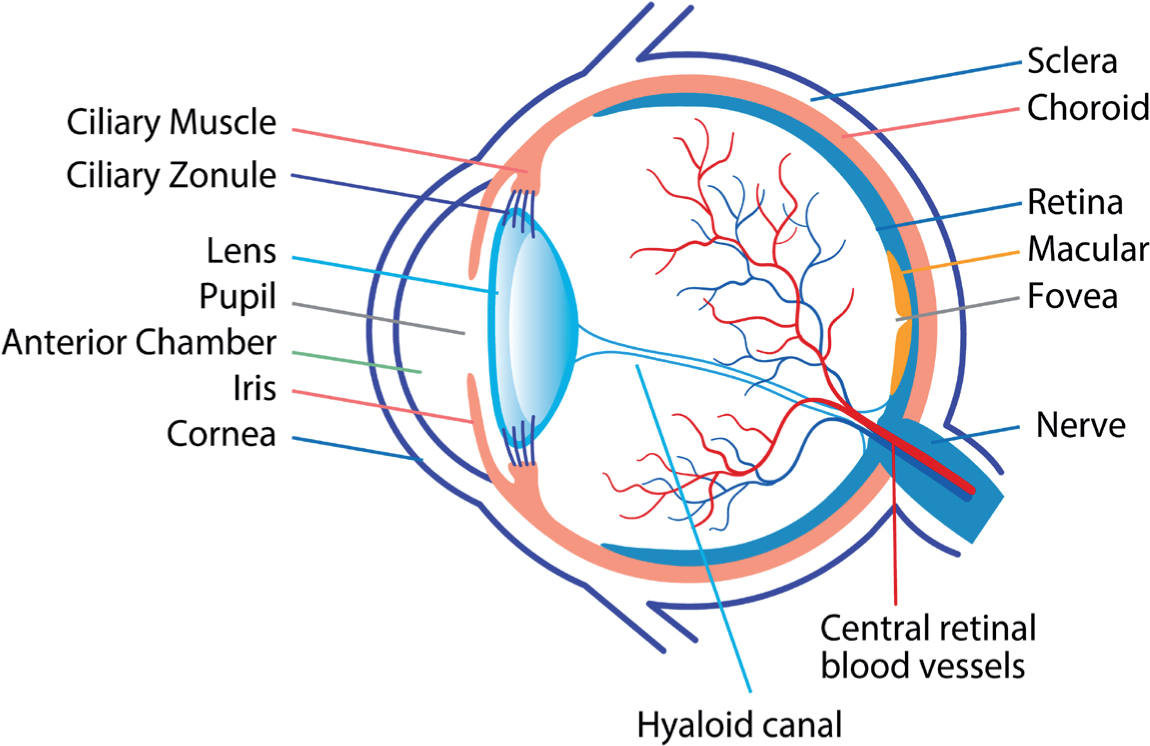
Aqueous humour supplies oxygen and nutrients to the cornea, lens and other cells lining the anterior chamber. Aqueous humour also helps maintain the eye's shape (Schehlein et al, 2017; Sharif, 2021). The ciliary body produces aqueous humour, which flows into the anterior chamber through the pupil (Schehlein et al, 2017).
The retina contains between six million and eight million cones, responsible for colour vision in bright light. Cones are concentrated in the fovea (Figure 1). The retina also contains 75 to 150 million rods, which are sensitive to low levels of light. Rods are dispersed all over the retina, with the exception of the optic disc (Marques, 2011). Nerves from the rods and cones feed into ganglion cells. Axons from the retinal ganglion cells merge to form the optic nerve, which leaves the eye through the optic disc (Jonas et al, 2017). After light reaches the retina, the brain takes between 0.15 and 0.30 seconds before perceiving an image (Marques, 2011).
Intraocular pressure
Between 70% and 90% of aqueous humour exits through a filter called the trabecular meshwork into the canal of Schlemm and, then, into the circulation (Sharif, 2021). The remainder drains through the uveoscleral pathway (Schehlein et al, 2017; Sharif, 2021). Usually, the amount of aqueous humour produced balances the amount flowing out (Sharif, 2021). This maintains the IOP within the (widely accepted normal range 10 mmHg to 21 mmHg) (Wang et al, 2018). Essentially, in people with COAG, the outflow pathway becomes restricted, due to, for instance, blockage of the trabecular meshwork with debris as well as age-related changes to cells and repair mechanisms. In closed-angle glaucoma, the iris blocks the anterior chamber. As such, aqueous humour cannot drain away (Jonas et al, 2017).
Several systemic factors including age, blood pressure and drugs used to treat comorbidities (Table 1) potentially influence IOP (Razeghinejad et al, 2011; Murray, 2018; Wang et al, 2018; Kang and Tanna, 2021; Wijnants et al, 2022). Glaucoma risk rises by 73% for each decade increase in age after 40 years, for example (Jonas et al, 2017). People of African ethnicity seem to be almost three times more likely to develop glaucoma than white Europeans (Gupta and Chen, 2016; Jonas et al, 2017). IOP also varies during the day. Each 1 mmHg rise in the circadian fluctuation in IOP increases the risk of progressive visual field loss by about 30% (Balendra et al, 2017).
A mismatch between aqueous humour production and outflow can increase IOP, which can, in turn, damage the retina. Each 1 mmHg rise in mean IOP increases the likelihood that ocular hypertension (raised IOP in an otherwise healthy eye) will progress to primary open-angle glaucoma by 10-11% (Schehlein et al, 2017; Weinreb, 2005). However, about half of patients with glaucoma have IOP in the normal range at diagnosis. This is known as ‘normal-tension glaucoma’ (Gupta and Chen, 2016; Schehlein et al, 2017).
Raised IOP beyond the intrinsic ocular defences against the pressure exerted by aqueous humour can damage many areas of the eye. For example, a depression at the centre of the optic disc, where there are no nerve fibres, is called the cup. Patients with glaucoma show an enlarged, often distorted, cup. Increased IOP also thins the layer of retinal nerves (Jonas et al, 2017; Greener, 2019) and can damage the lamina cribrosa, a layer of collagen that covers the optic nerve as it leaves the eyeball. This, in turn, undermines the structural and metabolic support of the retinal ganglion cells (Kang and Tanna, 2021). IOP fluctuations may undermine the mechanisms that protect retinal ganglion cells or cause chronic remodelling of the lamina cribrosa (Balendra et al, 2017).
Factors other than IOP
As mentioned, about half of glaucoma patients have ‘normal-tension glaucoma’. Moreover, some people with raised IOP do not develop glaucoma possibly because they have stronger defences against pressure. (Gupta and Chen, 2016; Schehlein et al, 2017). So, factors other than IOP seem to contribute to the risk of developing glaucoma, including abnormal ocular blood flow, intrinsic vulnerability of the lamina cribrosa, low intracranial pressure, autoimmunity and mitochondrial dysfunction (Balendra et al, 2017; Kang and Tanna, 2021).
Researchers have identified several factors that could contribute to normal-tension glaucoma. In people with normal-tension glaucoma, the nerves in the retina may be especially sensitive to damage from pressure. An abnormally high-pressure gradient across the lamina cribrosa, degeneration of the optic nerve sheath and reduced blood flow to the optic nerve could also increase the risk of normal-tension glaucoma (Greener, 2019; Jonas et al, 2017).
Diagnosis
Glaucoma is typically asymptomatic. Indeed, about half of people, even in economically developed countries, are unaware that they have glaucoma (Kang and Tanna, 2021). So, regular eye tests are important. Table 2 summarises the National Institute of Health and Care Excellence's (NICE, 2022) recommendations for diagnosis of COAG and related conditions.
Table 2. NICE's (2022) recommendations for diagnosis of COAG and related conditions
| Offer these tests | Establish severity at diagnosis by assessing visual field using standard automated perimetry (central thresholding test); repeat if necessary |
| Assess optic nerve and examine the fundus using a stereoscopic slit lamp with pupil dilatation; image the optic nerve head (eg with stereoscopic optic nerve head imaging or optical coherence tomography) at diagnosis as baseline | |
| Measure IOP using slit-lamp mounted Goldmann applanation tonometry | |
| Assess peripheral anterior chamber configuration and depth using gonioscopy or the van Herick technique if circumstances exclude gonioscopy (eg people cannot participate because of physical or learning disabilities) | |
| Measure central corneal thickness | |
| At diagnosis of ocular hypertension, assess the risk of future visual impairment, considering risk factors such as those right | IOP level |
| Central corneal thickness | |
| Family history | |
| Life expectancy |
By the time repeatable visual field defects emerge, glaucoma patients have probably lost 30–40% of their retinal ganglion cells (Rees et al, 2010; Gupta and Chen, 2016). Initially, patients may report blurred areas of vision. As retinal damage progresses, patients may report diffusely foggy or dark vision. In advanced glaucoma, patients may lose the ability to discriminate differences in light or report ‘tunnel vision’ (Kang and Tanna, 2021).
IOP typically rises rapidly in acute closed-angle glaucoma. As a result, people with acute closed-angle glaucoma may experience blurred vision, eye pain, red eye, cloudy corneas and halos, such as around lights, headache, nausea, vomiting and abdominal pain, which may be misdiagnosed as gastroenteritis (Murray, 2018; Sharif, 2021). Patients reporting these symptoms should be referred as medical emergencies (Murray, 2018).
Iatrogenic glaucoma
Glaucoma can arise as an iatrogenic complication following the use of certain systemic drugs that increase IOP (Table 1), eye surgery or after using lasers to treat retinal disease (European Glaucoma Society, 2017). Some estimates suggest that over-the-counter or prescription medicines cause at least a third of acute closed-angle glaucoma cases (Razeghinejad et al, 2011).
Systemic glucocorticoids, for example, can increase IOP. At high doses or in people with a family history of glaucoma, inhaled glucocorticoids may significantly increase IOP (Wijnants et al, 2022). A study from Singapore that analysed 8063 Asian people (mean age 57 years; 50.9% female) without glaucoma found that systemic beta-blockers were associated with a 0.45 mmHg lower IOP than controls. However, angiotensin-converting enzyme inhibitors (0.33 mmHg higher), angiotensin receptor blockers (0.40 mmHg higher), statins (0.21 mmHg higher) and sulfonylureas (0.34 mmHg higher) were associated with significantly raised IOP. Biguanides, insulin and alpha-glucosidase inhibitors were not associated with significant changes in IOP (Ho et al, 2017).
The study has certain limitations including measuring IOP at one point only, not being able to assess concordance, duration of use and dose, and enrolling people without glaucoma. Whether some of the changes are clinically relevant (Ho et al, 2017) and whether the results apply to other ethnic groups are moot. Nevertheless, given the range of drugs linked to iatrogenic glaucoma (Table 1) all HCPs need to be vigilant for this adverse event.
Pharmacological management
Currently, reducing IOP is the only management strategy for glaucoma (Ho et al, 2017; Storgaard et al, 2021). Broadly, three approaches lower IOP: laser trabeculoplasty, surgery and medication (Storgaard et al, 2021). NICE advocates 360° selective laser trabeculoplasty to treat newly diagnosed ocular hypertension with IOP of 24 mmHg or more for most people at risk of visual impairment within their lifetime. However, selective laser trabeculoplasty is not always suitable, such as in patients with pigment dispersion syndrome, and some people need additional treatment to reduce their IOP sufficiently to prevent the risk of visual impairment (NICE, 2022). Studies from Korea and the US report that 5.3% and 4.2% of patients with open-angle glaucoma undergo surgery within 5 years and 48 months of diagnosis respectively (Lee et al, 2022).
Considerations for prescribers
HCPs should check for relevant comorbidities or potential interactions before using any drug to treat ocular hypertension or glaucoma (NICE, 2022). Even experienced eye drop users often have difficulty correctly administering their treatment, a topic addressed below (Muir et al, 2022). HCPs also need to emphasise the importance of concordance (Cho et al, 2022; Muir et al, 2022), another topic addressed below.
Patients must be able to read and understand the packaging and instructions on their eye drops and other treatments. Therefore, HCPs should follow the Accessible Information Standard (NHS England, 2016) and, when appropriate, use large print or another reading format. If a patient reads Braille, ask pharmacists to ensure labels are not stuck over the embossed words (Greener, 2019).
In addition, benzalkonium, a widely used preservative in eye drops, can cause eye irritation and chronic ocular surface disease (Greener, 2019). NICE suggest offering preservative-free eye drops to people who have an allergy to preservatives or present with clinically significant and symptomatic ocular surface disease (NICE, 2022).
Several groups of drugs lower IOP. This section briefly introduces the main classes of drugs used to treat glaucoma. The section is not intended to be comprehensive. For further details, HCPs should read the summary of product characteristics and review the main studies for each drug.
Prostaglandin analogues
Prostaglandin analogues (eg bimatoprost, latanoprost, tafluprost and travoprost) are the first-line pharmacological treatment for glaucoma. NICE suggest offering a generic prostaglandin analogue to people with ocular hypertension if they are at risk of visual impairment within their lifetime and laser trabeculoplasty is not suitable, refused or the patient needs interim or additional treatment (NICE, 2022).
Prostaglandin analogues increase the outflow of aqueous humour through the uveoscleral pathway and typically reduce IOP by about 20–35% (Gupta and Chen, 2016; Schehlein et al, 2017). Prostaglandin analogues' side effects include pink eye and darkening of the iris (more common in those with green or hazel than blue eyes). Eyelashes may grow longer, thicker and darker (which may occur in up to three-quarters of people). Darkening of the eyelid pigmentation can occur in up to a quarter of users (Inoue et al, 2012; Law, 2010).
Sympathomimetics
Physostigmine (also called eserine), a sympathomimetic (cholinergic agonist), was the first drug used to treat glaucoma, introduced in 1876. Pilocarpine, another sympathomimetic, entered the clinic the following year (Storgaard et al, 2021). Sympathomimetics increase the outflow of aqueous humour through the trabecular meshwork and typically lower IOP by about 20-25% from baseline (Gupta and Chen, 2016; Schehlein et al, 2017). Sympathomimetics can cause side-effects that include brow ache, miosis, sweating, bronchospasm and diarrhoea (Sharif, 2021).
Carbonic anhydrase inhibitors
Carbonic anhydrase inhibitors prevent the interconversion of carbon dioxide and carbonic acid. This, in turn, reduces aqueous humour production by the ciliary bodies (Stoner et al, 2021). Topical carbonic anhydrase inhibitors (eg brinzolamide and dorzolamide) typically lower IOP by about 15-20% from baseline (Gupta and Chen, 2016; Schehlein et al, 2017). Adverse events include: irritation and hyperaemia of the surface of the eye and cornea, abdominal discomfort, aplastic anaemia, weight loss, crusty eyelashes, fatigue and a bitter taste (Glaucoma UK, 2020; Sharif, 2021).
Alpha-adrenergic agonists
Alpha-adrenergic (strictly alpha2 receptor) agonists (eg apraclonidine and brimonidine) reduce aqueous humour production as well as, possibly, increasing outflow. Typically alpha-adrenergic agonists lower IOP by about 20-25% (Gupta and Chen, 2016; Schehlein et al, 2017; Glaucoma UK, 2020). A combination of brinzolamide and brimonidine is available (Glaucoma UK, 2020).
Side effects of alpha-adrenergic agonists include hyperaemia, dry mouth, contact dermatitis, tiredness, general weakness, apnoea and hypotension (Glaucoma UK, 2020; Sharif, 2021). Tachyphylaxis can mean that efficacy declines with long-term use (Sharif, 2021). Occasionally, alpha-adrenergic agonists cause severe allergic reactions. HCPs should advise patients that if one or both eyes becomes increasingly red, sore and sticky, even several months after starting treatment, they should consult an ophthalmologist or GP urgently (Glaucoma UK, 2020).
Beta-blockers
Beta-blockers (eg betaxolol, carteolol, levobunolol and timolol) reduce production of aqueous humour and typically lower IOP by about 20-25% from baseline (Gupta and Chen, 2016; Schehlein et al, 2017). Beta-blockers' side effects include: punctate keratitis (scattered, small areas of corneal damage), corneal anaesthesia, bronchospasm and bronchoconstriction, exacerbations of heart block, slow pulse (bradycardia), dizziness, tiredness, hypotension, depression and loss of libido or impotence (Glaucoma UK, 2020; Kido et al, 2022; Sharif, 2021). Combination drops are available that contain timolol and a drug from another class such as: brinzolamide, brimonidine, bimatoprost, dorzolamide, latanoprost, tafluprost and travoprost (Glaucoma UK, 2020).
The importance of concordance
Concordance with pharmacological treatments for glaucoma ranges from 10% to 83% (Muir et al, 2022). Cho et al (2022) used electronic monitoring to assess concordance (the system recorded each time the bottle was opened) among patients who admitted poor concordance. Patients took a median of 74% of the doses over three months. Of these patients, 56% were nonadherent (defined in this study as using 80% or less of the doses), assuming of course bottle opening translates into dosing (Cho et al, 2022).
The authors compared various assessments of concordance based on pharmacy data and self-assessment. Asking ‘Over the past month, what percentage of your drops do you think you took correctly?’ most accurately (71% accuracy; 84% sensitivity) predicted electronically monitored non-concordance (Cho et al, 2022).
HCPs should stress that good concordance slows disease progression and preserves sight. A study from the US followed 6343 patients with open-angle glaucoma for up to 12.9 years (mean 5.8 years). Average treatment concordance was 73%. A mathematical model suggested that in people with moderately severe glaucoma at baseline, the time to progression was 8.7 years for 20% concordance. However, 80% concordance delayed glaucoma progression by a year (Shu et al, 2021).
Education of glaucoma patients and caregivers who remind people to take their medication or administer the eye drops can significantly improve concordance. In one study, glaucoma patients, and if applicable caregivers, received an intervention that included glaucoma education, personalised disease suggestions (eg administration aids) and a reminder aid. Controls received education about general eye health. The participants admitted poor concordance before the study. Nevertheless, the intervention significantly (p< 0.0001) increased the mean proportion of prescribed doses taken on schedule over six months, assessed using electronic monitoring, compared with controls (85% and 62% respectively) (Muir et al, 2022).
Administering eye drops
Patients (or their carers) can encounter difficulty administering eye drops, if, for example they have joint issues in their hands, which can contribute to poor concordance. HCPs should consider whether a patient would benefit from an aid that supports positioning, squeezing the bottle or both. Several are available including Opticare, Opticare Arthro, Autodrop and Autosqueeze (Glaucoma UK, 2020). Glaucoma UK and the Royal National Institute of Blind People offer patients and caregivers further advice about administering eye drops and other practicalities of living with ocular disease.
Punctal occlusion slows the rate at which the drop drains through the tear duct and into the throat. The patient should close their eye gently after instilling the drop. The patient should then gently presses on the inside corner of the eye, by the nose, with a finger for one to two minutes (Glaucoma UK, 2020; Greener, 2019). Punctal occlusion also reduces the risk of experiencing an unpleasant taste, which can be a side effect of some glaucoma drugs, such as alpha-adrenergic agonists and carbonic anhydrase inhibitors (Gupta and Chen, 2016; Bancroft et al, 2019).
New treatments
Despite the range of drugs, one in seven people treated for glaucoma were blind in one eye within 20 years (Gupta and Chen, 2016); there is a pressing need for new treatments. Researchers are, for instance, developing drugs that bind specifically to particular types of carbonic anhydrase, called isoforms (Stoner et al, 2021). Other possible new treatments include adenosine receptor agonists (pilocarpine may act partly through this mechanism) and prostanoid receptor agonists (prostanoid refers to the class of biological mediators that includes prostaglandins) (Balendra et al, 2017; Kaufman et al, 2018; Schehlein et al, 2017). Ripasudil, a topical Rho kinase inhibitor inhibits contraction of the trabecular meshwork, which decreases IOP (Balendra et al, 2017; Kaufman et al, 2018; Schehlein et al, 2017).
Currently, reducing IOP is the only management strategy for glaucoma (Ho et al, 2017; Storgaard et al, 2021). However, several drugs in development seek to bolster nerves' ability to withstand IOP. For example, excessive stimulation of the N-methyl-D-aspartate (NMDA) receptor by the excitatory neurotransmitter glutamate allows high levels of calcium to enter cells. This may contribute to nerve death in people with glaucoma (Schehlein et al, 2017). NMDA antagonists may, therefore, protect retinal ganglion cells (Balendra et al, 2017; Sánchez-López et al, 2018).
Calcium channel blockers seem to improve vision in people with glaucoma by increasing blood flow to the optic disc and may protect retinal ganglion cells from apoptosis (controlled cell death) and necrosis (Balendra et al, 2017). Neurotrophins – such as nerve growth factor, brain-derived neurotrophic factor and ciliary neurotrophic factor – reduce levels of free radicals (oxidative stress) and lowering immune activity, which can promote neuronal survival (Kang and Tanna, 2021).
Conclusions
Glaucoma is the most common cause of irreversible visual loss worldwide (Sun et al, 2022). Early diagnosis and rapid treatment is essential to preserve sight (Hamid et al, 2022). IOP is, currently, the only modifiable risk factor for glaucoma, although drugs targeting other pathways are being developed (Ho et al, 2017; Greener, 2019; Storgaard et al, 2021).
By the time repeatable visual field defects emerge, glaucoma patients have probably lost 30-40% of their retinal ganglion cells (Rees et al, 2010; Gupta and Chen, 2016). So, regular eye tests and considering if glaucoma could be an iatrogenic complication are important.
Broadly, three approaches lower IOP: laser trabeculoplasty, surgery and drugs (Storgaard et al, 2021). Prostaglandin analogues are the first-line pharmacological treatment. HCPs should consider whether a patient would benefit from an aid that supports positioning, squeezing the bottle or both (Glaucoma UK, 2020). The prospects for a new generation of glaucoma drugs seems promising. In the meantime, the growing elderly population and increasing pressure on outpatient ophthalmology clinics means that primary care HCPs can expect to have a greater role in eye care. While this poses challenges, improved community care and greater awareness among HCPs generally could help save more patients' sight (Greener, 2019).


