In 2010, 6.3 million people worldwide were known to be either blind or visually impaired due to glaucoma (Bourne et al, 2016). In the UK, glaucoma accounts for approximately 11% of severe sight impairment registrations (Quartilho et al, 2016). The high level of visual morbidity can be party attributed to the absence of symptoms until the late stages of the disease coupled with the irreversible nature of glaucoma damage, which typically occurs at the periphery before gradually encroaching onto the central visual axis. The disease tends to progress insidiously over years, but there is substantial variation between individual rates of progression. The disease burden is responsible for a massive socioeconomic cost and accounts for over one million NHS visits each year (Azuara-Blanco et al, 2007). A typical visit involves automated visual field analysis, a consultation, followed by a slit-lamp examination including intraocular pressure (IOP) measurement and optic disc assessment.
Pathophysiology
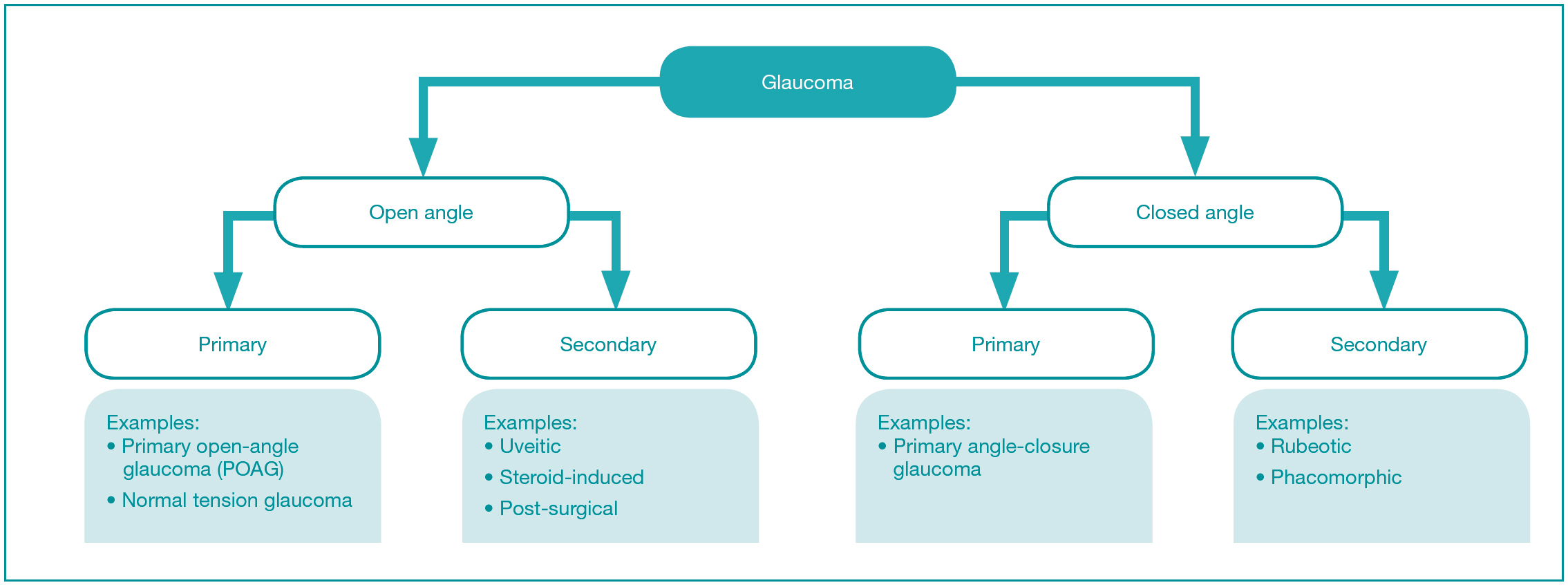
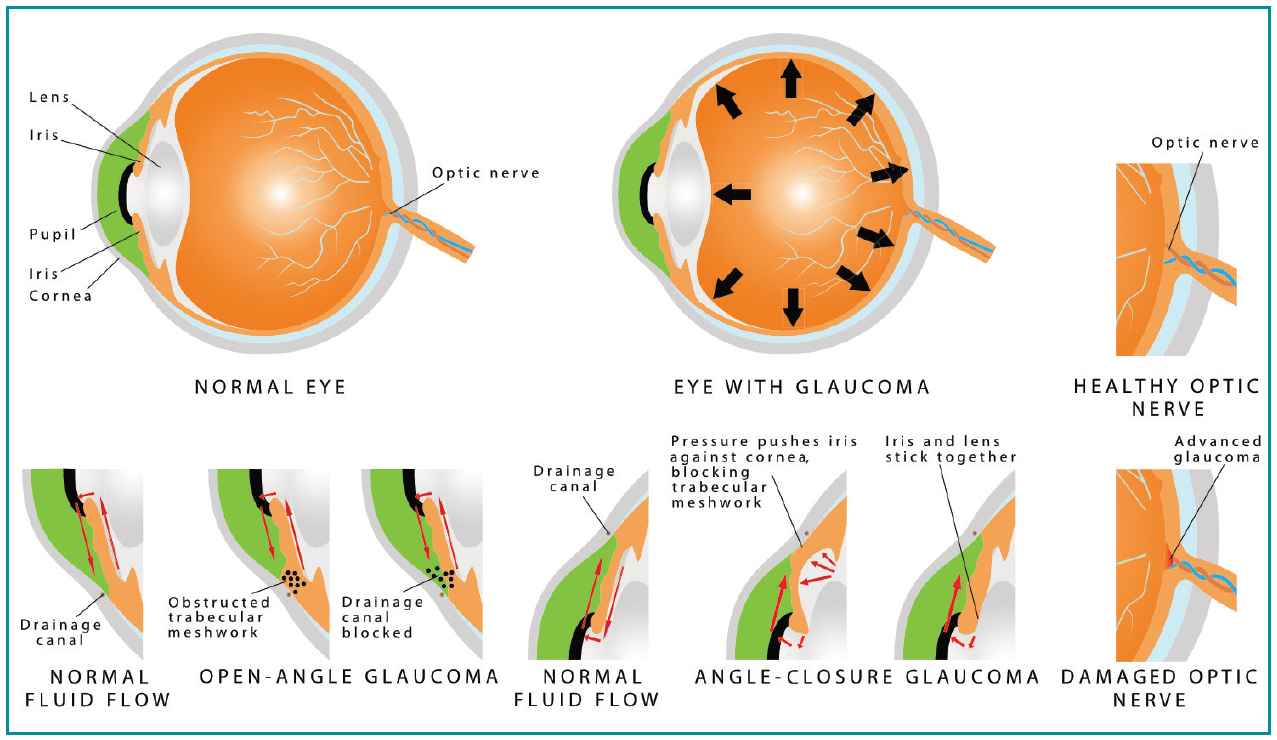
There are various ways to classify the different types of glaucoma (Figure 1). The most common type of glaucoma is open-angle or angle-closure glaucoma (Figure 2). The ‘angle’ is the name given to the junction between the outer edge of the iris and the inner cornea, which contains a thin circular band of tissue called the trabecular meshwork, which is responsible for draining the fluid produced by structures behind the iris. If there is a clear path for the fluid to reach the trabecular meshwork, this is called an ‘open angle’, but if there is a physical obstruction to the fluid path, such as by iris tissue, this is called a ‘closed angle’. The other main types of glaucoma are primary or secondary glaucoma. The latter name is used when there is a known underlying cause for the glaucoma, such as inflammation (uveitis), steroid treatment-induced, previous eye surgery or diabetes-related neovascularisation. Primary open-angle glaucoma (POAG) is the most common type of glaucoma, but the exact cause is not well understood. It is thought to be due to microscopic changes in the trabecular meshwork causing increased resistance to fluid drainage. In all types of glaucoma, there is death of the nerve cells signalling from the eye to the brain (retinal ganglion cells). Raised pressure in the eye is the most important causative factor, but genetic and environmental factors are also responsible (Evangelho et al, 2019). Pathological progressive loss of the retinal ganglion cells and their corresponding nerve fibres, which project to the brain via the optic nerve, leads to a ‘cupped’ appearance of the optic nerve head with corresponding functional visual field defects.
Risk factors
The only currently modifiable risk factor for glaucoma is IOP, with the normal range between 10–21 mmHg. If the IOP is consistently above 21 mmHg with no evidence of optic nerve damage or visual field defects, the diagnosis is ocular hypertension (OHT). Patients with OHT have a low, but significant risk of developing glaucoma, which is reduced with sufficient lowering of the IOP (Gordon et al, 2002). Conversely, normal-tension glaucoma (NTG) is the presence of glaucomatous features with IOP consistently within the normal range. In these patients, IOP reduction remains effective, but their glaucoma can progress even at low pressures (Anderson, 2003). In POAG, the risk of glaucomatous progression is approximately halved with IOP-lowering treatment and the protective effects continue in a tightly linear fashion with IOP reduction. Additional poor prognostic factors are age and severity of visual field defect on presentation (Leske et al, 2003). Prevalence of glaucoma types vary significantly with ethnicity, for example in POAG, a large study showed rates of 1.41%, 2.14% and 4.23% in Asian, white and black people respectively (King et al, 2013) Myopia (short-sightedness) and a family history of glaucoma are also associated with an increased risk of developing glaucoma (King et al, 2013).
Treatment
The National Institute for Health and Care Excellence (NICE) (2017) glaucoma guidelines suggest withholding treatment for patients with OHT who are not at risk of visual impairment in their lifetime and patients with suspected glaucoma but with an IOP less than 24 mmHg. Instead, suitable long-term observation for new features of glaucoma is warranted. In cases of confirmed glaucoma, the advice is to offer treatment.
Target IOP
The aim of glaucoma treatment is to sufficiently slow the rate of disease progression to maintain visual function while maximising quality of life. This can usually be achieved by lowering the IOP to a personalised target, which is determined by a combination of a patient's life expectancy, risk factors and monitoring of their glaucoma progression rate (Bryan and Crabb, 2018). Even if the target IOP is being achieved, it should be regularly reviewed and lowered if there is evidence of glaucomatous progression. All medical interventions are associated with a varying degree of reduced quality of life, particularly lifelong treatment such as eye drops; therefore, the minimal intervention possible to achieve the target IOP should be a mantra of treatment.
Categories of treatment
Lowering IOP can be achieved by one, or a combination of: long-term medication (usually in the form of eye drops), laser and surgery. The majority of patients are initially treated with eye drops and most never require surgery, but early glaucoma surgery is an option in patents who present with advanced visual field loss (Musch et al, 2009). Glaucoma surgery is usually reserved for patients where medical therapy alone is deemed insufficient to adequately control their glaucoma. The precise role of selective laser trabeculoplasty (SLT) as initial monotherapy remains unclear and we await the publication of the results of a large trial that aimed to establish its effectiveness compared to medication (Garg and Gazzard, 2018). Primary angle-closure can often be treated by altering the anatomy with either a laser peripheral iridotomy (a small hole in the iris), or in select cases with IOP above 30 mmHg, by performing early cataract surgery (Azuara-Blanco et al, 2016).
Principles of prescribing glaucoma medications
There are several classes of glaucoma medication available to prescribe alone or in combination, which target different parts of the drainage pathways to lower IOP (Table 1). To date, there is no medication proven to be directly neuroprotective to the retinal ganglion cells (Sena and Lindsley, 2017).
The most effective medications in terms of IOP-lowering are the prostaglandin analogues closely followed by beta-blockers, which guides their use as first- and second-line choices, respectively, as initial monotherapies (van der Valk et al, 2005). For all glaucoma medication, a larger absolute reduction in IOP is observed when the baseline pre-treatment IOP is higher. If the target IOP is not reached with a monotherapy, or the first-line drug is not tolerated, the next step is either to switch to a different monotherapy or combine a second class of glaucoma medication, which often have a synergistic effect compared with either alone. There are fixed formulations of common combinations (Box 1), which avoids the reduction in adherence to treatment seen with more complex treatment regimens (Olthoff et al, 2005). If two agents are insufficient to reach the target IOP, the next step is to either add a third class of medication or proceed to glaucoma surgery (NICE, 2017).
Table 1. Summary of glaucoma medication
| Class and mode of action | Example drugs and dose | Example contraindications | Significant side effects |
|---|---|---|---|
| Prostaglandin analogues:Increase outflow pathway by absorption | Latanoprost 0.005%† | Active intraocular inflammationActive herpetic keratitisContact lenses (can be reinserted after 15 minutes) | Increased pigmentation of the iris and periocular skinIncreased eyelash growthDry eye symptomsConjunctival rednessMacular oedemaOrbital fat atrophy |
| Bimatoprost 0.03%† | |||
| Travoprost 0.03% | |||
| Dose: usually once at night | |||
| Beta-blockers:Decrease fluid production | Timolol 0.1%†, 0.25%, 0.5% | Chronic obstructive pulmonary diseaseAsthmaBradycardiaHeart failure | Dry eye symptomsConjunctival rednessAllergic conjunctivitisBradycardiaHypotensionAsthma exacerbationDepressionSexual dysfunction |
| Levobunolol 0.25% | |||
| Betaxolol 0.5% | |||
| Dose: once or twice a day | |||
| Carbonic anhydrase inhibitors:Decrease fluid production | Dorzolamide 2%† (topical) | Topical: corneal oedema | Tingling in lips and limbsNausea and vomitingTaste disturbanceTinnitusKidney dysfunctionBone marrow dysfunction |
| Brinzolamide 1% (topical) | |||
| Topical dose: 2 or 3 times a day | |||
| Acetazolamide (oral)Oral dose: 125–250 mg, 1–4 times a day | Oral: renal dysfunction, kidney stones, low sodium or potassium | ||
| Alpha-2 adrenergic agonists:Decrease fluid production | Apraclonidine 0.5%, 1% | Age under 18 yearsVery low BMIOral monoamine oxidase inhibitor use | Allergic conjunctivitisContact dermatitisLid retractionDry nose and mouthHypotensionBradycardiaFatigue |
| Brimonidine 0.2% | |||
| Dose: 2 or 3 times a day | |||
| Parasympathomimetics:Increase outflow pathway by mechanical opening of the trabecular meshwork | Pilocarpine 0.5–4% | Intraocular inflammationNeovascular glaucomaPeptic ulcerRecent myocardial infarctionHypotensionParkinsonismEpilepsy | Blurred vision due to pupil constrictionConjunctival rednessRetinal detachmentCataractHeadacheWheezingBowel cramps |
= preservative-free preparation available
Box 1.Common fixed combination formulationsBimatoprost 0.03% and Timolol 0.5%† (Ganfort)Latanoprost 0.005% and Timolol 0.5%† (Xalacom, Fixapost)Dorzolamide 2% and Timolol 0.5% (Azarga)Brinzolamide 1% and Brimonidine 0.2% (Simbrinza)†= preservative-free preparation available
Pregnancy and breastfeeding
There is a clinical challenge in managing glaucoma in pregnancy, breastfeeding and in women wishing to conceive. All known glaucoma medications have either been shown to be teratogenic or have no available safety data in pregnant and breastfeeding women (Razeghinejad et al, 2011). Such patients are best managed by an experienced ophthalmologist, but a general approach is to weigh up the risks and benefits of treatment on a case-by-case basis with a preference for non-medicinal forms of treatment.
Preservative-free eye drops
Ocular surface disease (OSD), which comprises primarily evaporative dry eye and blepharitis (eyelid margin inflammation) can be precipitated by using topical glaucoma medication, or if OSD is already present, can be exacerbated by it. In addition to the cumulative effect of the active drug compounds, preservatives such as benzalkonium chloride (BAC) have also been shown to worsen OSD (Rossi et al, 2013). In addition to treating the OSD directly, to relieve the effect of BAC, there are preservative-free versions of a number of commonly used single and fixed-combination topical glaucoma medications. The 2017 NICE guidance for glaucoma suggests offering preservative-free eye drops if there is evidence that the patient is allergic to the preservative or has clinically significant and symptomatic ocular surface disease.
Adherence to treatment
Adherence to any prescribed treatment is essential for the maximum therapeutic effect. Glaucoma drop adherence has been extensively studied and is considered to be an issue in a significant proportion of patients, although the true figure is not known. Non-adherence can manifest in various ways, including missed doses, incorrect drops being used and incorrect application of the medication. Tsai et al (2003) described four categories associated with non-adherence to glaucoma medication, which include both volitional and non-volitional factors (Table 2). If a specific reason for non-adherence has been identified, the clinician can employ measures in an attempt to improve adherence. As a general rule, review appointments should include non-accusatory queries to the patient about their recent adherence to treatment. It should not be presumed that patients are knowledgeable about the technique of instilling eye drops and detailed instruction should be given on correct administration. Patients with physical barriers to instilling drops, such as arthritis of the hands, can be provided with compliance devices (Moorfields Eye Hospital NHS Foundation Trust, 2018). The International Glaucoma Association (IGA) (2017) offers a free online leaflet describing a range of different eye drop dispensing aids, some of which may be prescribed, with a large number are available from the IGA. There are generic versions of many glaucoma medications, which are often prescribed for cost considerations. A change from branded to generic drops or between the various generic drops can mean different packaging, bottle type, preservative quantity and storage requirements. This can lead to confusion and suspicion by patients and to a possible decrease in adherence (Dubois, 2013). If a patient is not meeting their target IOP, or if the response to a recent change in treatment has not shown the anticipated effect, non-adherence should be suspected and investigated before a new management plan is considered. There are myriad of sustained-release novel drug delivery technologies under development, which should have a positive impact on levels of adherence when they become available (Varma, 2018).
Table 2. Categories associated with non-adherence to glaucoma medication
| Situational / environmental | Major life event, busy lifestyle |
|---|---|
| Patient | Poor understanding of condition, significant comorbidities, memory problems, self-neglect |
| Medication | Cost of treatment, side effects, difficulty obtaining medication |
| Provider | Complex treatment regimen, poor communication with clinician |
Conclusion
In established cases of glaucoma, treatment is necessary to limit the amount of visual morbidity affecting patients over their lifetime. The minimal level of intervention necessary to achieve this aim should minimise the associated quality of life reduction from therapy. This can be achieved through a stepwise escalation of treatment with a tailored IOP target, allowing identification of the most at-risk patients. Finally, the management plan must be acceptable to the patient, as only with collaboration and proper adherence will the best outcomes be reached.
Key Points
- In patients diagnosed with glaucoma, it is important to ensure the correct type of glaucoma is diagnosed as the treatment choices can differ
- During treatment, decide on a target intraocular pressure individual for each patient and be prepared to adjust the target value depending on monitoring of glaucoma progression during follow-up visits
- Prescribe glaucoma medication in a stepwise fashion, taking into account any contraindications and informing patients about possible side-effects
- The aim of glaucoma treatment is to maximize overall quality of life by maintaining visual function using the minimum necessary intervention
CPD reflective questions
- What is the generally accepted normal range of intraocular pressure in an average population?
- How would you answer a patient who is asking why they are being offered treatment for ocular hypertension, even though it means that they do not have glaucoma?
- Which class of glaucoma medication is contraindicated in patients with asthma?
- Which other pressure-lowering treatment is suitable for some patients other than medication or surgery?
- What reasons could there be for a patient not showing a response to a change in their treatment at the next follow-up appointment?


