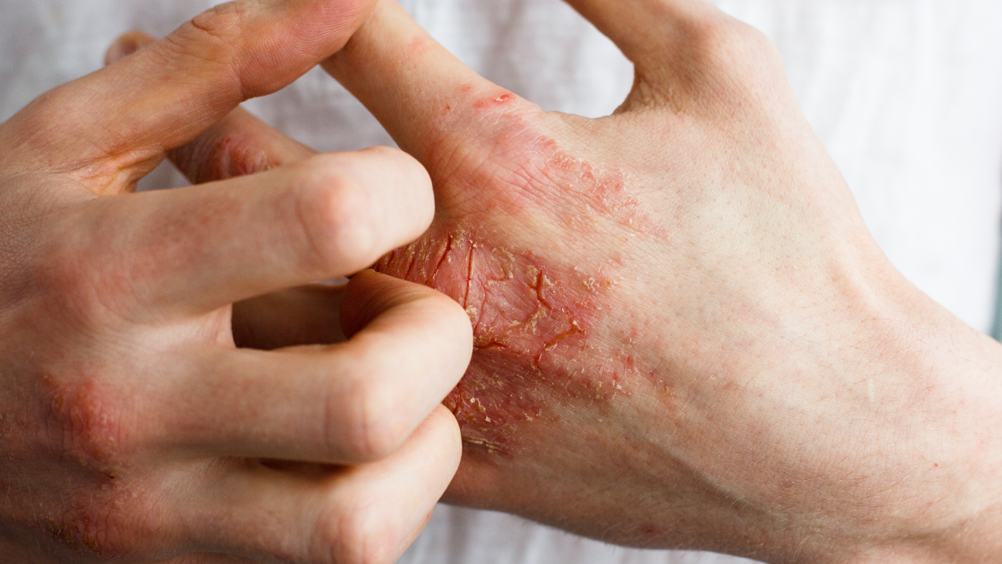
This article will examine the guidance and evidence for prescribing topical treatments in eczema management. Prescribers will assess patients with eczema and should manage symptoms according to national guidance. NICE provides clinical knowledge summaries and specific guidance for children with atopic eczema under 12 years of age (National Institute of Health and Care Excellence (NICE), 2007; 2018). The Primary Care Dermatology Society (PCDS) provides eczema treatment pathways with practical advice for managing adults and children with eczema in primary care (PCDS, 2019). An overview of the PCDS eczema treatment pathways are outlined in Box 1. This article will explore the benefits and risks of the five main topical treatment strategies for eczema: emollients, topical corticosteroids, topical immunomodulators, antimicrobials and antihistamines.
Box 1.Management ABC
- A. Avoid triggers; soaps or anything that lathers, cigarette smoke, irritant clothing
- B. Bland moisturisers; an absolutely essential part of treatment. Should be fragrance-free. Applied ideally 3-4 times a day; prescribe adequate quantities (at least 500 g/week); patient choice improves concordance; bath additives are not recommended; use emollients to wash (apply before wetting the skin). Ideally, wash hair over the sink to avoid shampoo on skin causing irritation
- C. Control inflammation
- Topical steroids matched to the severity and anatomical site – mild (face and flexures), moderate, potent
- Topical steroids use once daily for 1-6 weeks until settled, decreasing to twice weekly use for maintenance if frequent flares
- Step-up use to daily during a flare, then wean back down for maintenance therapy (reduces frequency of flares)
- Adults: Calcineurin inhibitors (such as topical tacrolimus or pimecrolimus) are useful as second-line and particularly useful in delicate sites (eyelids, face, flexures)
- Children (0-12years): Topical calcineurin inhibitors are useful as second-line treatment. Tacrolimus 0.1% (off-license) can be used for children and is particularly useful when applied to delicate sites i.e. flexures, eyelids
- There is unjustified topical steroid phobia amongst healthcare professionals; there is robust evidence of the safety in long-term use in eczema.
Source: NICE, 2007
Emollients
Emollients are first-line therapy in eczema, this is evidence-based UK guidance (NICE 2007; 2018; PCDS, 2019). Complete emollient therapy should always be advised, this is: ‘everything that goes on the skin should be emollient-based, and all soaps and detergents should be replaced with emollient wash, bath and shower products (NICE, 2007; Cork and Danby, 2009). Most emollients can be used as soap substitutes and eave-on emollients.
Benefits
The benefits of complete emollient therapy, even when eczema is clear is well documented, as evidence shows appropriate emollient use will reduce eczema flares (inflammation) and the need for other treatments (Grimalt et al 2007; Wiren et al, 2009). Emollients have several key actions to prevent dry skin and repair the skin barrier, as genetic filaggrin mutations result in a life-long abnormal skin barrier (Palmer et al, 2006). Emollients act by occluding transepidermal water loss (TEWL) with a surface film of lipids; this increases water in the stratum corneum, which essentially restores barrier function, preventing the entry of environmental triggers (Cork and Danby, 2009).
Emollients can only be beneficial if they are prescribed in adequate quantities, with informed patient choice (including choice of formulation) and support on adherence (including application technique) (Moncrieff et al, 2014). The correct application should be demonstrated to the patients, this is application in long soothing downward strokes in the direction of hair growth-this application method prevents follicultis There should be a time interval of at least 30 minutes between the application of topical flare treatments (topical corticosteroids, topical immumodulators - pimecrolimus only) and emollient (Moncrieff et al, 2014). The exception is tacrolimus ointment, which should not be applied to skin within two hours of the application of an emollient cream (NICE, 2004).
All patients with eczema should be prescribed at least one emollient; adults require 500 g per week and children, 12 years and under, a minimum of 250 g per week (NICE, 2007; PCDS, 2019).
The Bath Additives in the Treatment of Childhood Eczema (BATHE) study in children with atopic eczema found that emollient bath additives do not add any benefit over standard management (Santer et al, 2018). However, it is important to wash with soap substitutes, so depending on patient preference, bath additives may still be a wash emollient of choice for some patients. The British Association of Dermatologists (BAD) (2018) position statements adds: ‘By not allowing routine prescribing of bath/shower preparations, an entire type of emollient preparation is being restricted in people with eczema–this is different from removing/streamlining different formulations/products within a type of emollient preparation’ (BAD, 2018).
Risks
The main risks of emollients are flammability and slipping hazards. Emollients are generally paraffin-based, while the products are not flammable in themselves, they can act as an accelerate increasing the speed of ignition and intensity of the fire when fabric with residue dried on it is ignited (Medicines and Healthcare products Regulatory Agency (MHRA), 2018). This evidence has been cited by case reports from fire and rescue services, where emollients have been known to have been used by victims or were present in the house at the time of the fire. The MHRA (2018) has issued safety guidance and advises that prescribers must instruct patients ‘not to smoke or go near naked flames because clothing or fabric such as bedding or bandages that have been in contact with an emollient or emollient-treated skin can rapidly ignite’. This advice applies to all paraffin based emollients regardless of paraffin concentration, all packaging now carries an alert sign. Emollients, particularly bath oils can cause slip hazards, advise patients to, use bath mats, clean baths and showers after use and to moisturise standing on a non-slip mat.
Constrained patient choice of emollients and formulations (lotions, creams, ointments or gels) can be a risk, it is important for prescribers to understand that there are no generic emollients. Emollients, which are prescribed on, cost alone means they may not suit the patient or be used effectively to control and prevent eczema flares. A practical example is that a patient prescribed high percentage paraffin ointment, may be cost-effective on formulary but will be cosmetically unacceptable and may lead to underuse or waste; whereas a humectant emollient creams contain NMFs (for example urea, glycerol) will produce similar rehydration as grease-based emollients with a higher degree of cosmetic acceptability and no waste (Cork and Danby, 2009). Some formulations can cause harm to eczematous skin, a well-reserached example is Aqueous cream BP has a simple formulation of, water, white soft paraffin and SLS (anionic surfactant). The MHRA in the light of evidence recommends that Aqueous cream be avoided, both as a leave-on emollient and as a washing product (MHRA, 2014). SLS, is a strong skin irritant and studies have shown that the application of aqueous cream BP weakens the epidermal barrier and actually increases trans-epidermal water loss in children with atopic eczema and additionally in healthy adult skin (Cork et al, 2003; Danby et al 2011).
Topical corticosteroids
Topical corticosteroids are the mainstay of eczema treatment, used occasionally or regularly to manage inflammatory eczema flares. The potency of topical corticosteroid prescribed should be based on the age of the patient, area of the body, severity of eczema and dilution should be avoided (NICE, 2018; 2020; PCDS, 2019). There are four groups of topical steroid in the UK:
- Group 1: mild – such as hydrocortisone 0.1%, 0.5%, 1%
- Group 2: moderate – such as clobetasone butyrate 0.05%, betamethasone valerate 0.025%
- Group 3: potent – such as betamethasone valerate 0.1%, fluocinonide 0.05%
- Group 4: very potent – such as clobetasol propionate 0.05%, diflucortolone valerate 0.3%
Benefits
Topical corticosteroids are beneficial if used correctly, generally for short treatment bursts. Patients often use a steroid for a few days with good effect and then immediately stop, and often the eczema flare recurs, these patients are then stalled in a sub-acute flare that does not easily resolve. This describes a sub clinical state, when the erythema (redness) has resolved, eczema is still active (Tang et al, 2014), therefore a longer treatment course, which is stepped down (either by potency or by days – moving from daily to alternate days to ‘weekend therapy’). Evidence from large systematic reviews is that mild to moderate potency steroids pose little or no risk to patients when used appropriately (Ponnambath and Pynn, 2014). The potential for topical steroid side effects can be minimalised by short-term flare treatment with once a day application, which is as effective as twice-daily and reduces the amount of topical steroid applied (Green et al, 2004). ‘Weekend therapy’ is recommended for adults and older children with more severe eczema, who frequently relapse, may be recommend proactive treatment, which involves using moderate or potent topical steroids on two consecutive days a week (hence the term ‘weekend therapy’) to prevent flares (Berth-Jones et al, 2004).
Patients often find it hard to self manage eczema flares due to uncertainly and occasionally fear about the correct use of topical steroids. A barrier to effective to self-management of eczema is ‘steroid phobia’, a known phenomenon where patients often apply too little owing to unfounded fears concerning side effects (Charman et al, 2000). Patient's may be concerned about rebound, as it is common for patients to report that effective topical corticosteroid treatment can be followed by a flare of disease on withdrawal of treatment, or in the long term topical corticosteroids loss effectiveness (Hengge et al, 2006). Dermatologists explain this as tachyphylaxis, in the context that when corticosteroids are reduced in potency, disease activity flares (Hengge et al, 2006). In addition, eczema is often not treated adequately it can remain in a sub-acute state, in which the erythema (redness) has dissipated, but the eczema remains active - this sub clinical level of eczema is not easy to resolve, which indicates the need for weekend therapy or topical immunomodulators for maintenance treatment (Tang et al, 2014). Generally, side effects only occur if there is incorrect or excessive use, usually with prolonged use of potent or very potent steroids, which is discussed in the next section on risk.
Prescribers can improve benefits of topical corticosteroid treatment by advising patients on the correct technique for applying topical steroids in therapeutic doses, once a day. The fingertip unit (FTU) is a practical guide, the amount of ointment or cream expressed from a 5 mm nozzle tube, applied from the distal finger crease to the tip of the palmer aspect of the index finger. One FTU will cover an area equivalent to two flat hand areas of the affected area of eczema (Long and Finlay, 1991). The outdated direction ‘use sparingly’ often results in under-dosing and potentially confounds fear, the current application direction currently advised by the Joint Formulary Committee (2018) is ‘apply thinly’.
Risks
The risk of side effects topical corticosteroids needs to be kept in perspective, and the benefits for the majority of patients outweigh the risks. Side effects of topical corticosteroids relate directly to individual patients and potency. Local side effects at application sites concern skin atrophy, other local side effects include allergy, the risk of promoting infection and acneform eruptions (face, chest and back) and ophthalmic complications (Berth-Jones, 2016). Systemic side effects involve Inhibition of the pituitary-adrenal axis, which can result in adrenal suppression and Cushings Syndrome. However, these severe clinical problems are very rare compared to systemic corticosteroids and generally linked to potent and very potent topical steroid overuse (Hengge et al, 2006). Prescribers should be aware that excess glucocorticoids may lead to short stature due to suppression of growth hormone releasing hormone and growth hormone release from the hypothalamus and pituitary respectively, hence prescribing potency according to age and body surface area (Hengge et al, 2006).
The most common side effects from topical corticosteroids are local, with skin atrophy, involving both the epidermis and dermis being the most common. Prescribers need to prevent skin atrophy by individual prescribing according to the age of the patient, body site, potency of topical steroids and the vehicle (Hengge et al, 2006). Epidermal atrophy may be reversible but dermal atrophy is permanently caused by decreased fibroblast growth and reduced synthesis of collagen, the most susceptible area are skin flexures, due to thinner skin and increased moisture, and occluded areas of skin (Dhar et al, 2014). These risks can be reduced with once daily application, a study in 2007, recommended that topical corticosteroids are as effective applied once a day, as twice a day (Williams, 2007).
Prescribers should ensure once daily direction is advised to patients and including on the label.
Contact allergy to corticosteroids can occur, in one large study, 4.9% had an allergy to corticosteroid molecules, with hydrocortisone being the most common (Burden and Beck, 1992). Prescribers should be aware of this potential risk if eczema does not respond to treatment, however, this study is dated and was performed in adults. Risks of topical steroids include the exacerbation of skin infections. Prescribers should be aware of assessing for signs of skin infection (bacterial and viral), and treating accordingly, which will be discussed in the section on antibiotics and antibacterials.
Treating facial eczema with topical corticosteroids presents more risk than other areas of the body. Facial eczema should be diagnosed, as the incorrect treatment of other conditions that cause facial redness, such as rosacea can exacerbate and cause peri-oral dermatitis (Berth-Jones, 2016). This can pose difficult prescribing decisions, if patients have more than one facial skin condition, for example, acne and eczema. Topical steroids should be avoided for co-existent skin conditions and topical calcineurin inhibitors are a preferable prescribing choice to treat eczema alongside acne or rosacea.
The eyelids and around the eyes are very susceptible areas to the risks of topical corticosteroids. In ophthalmic's, it is well documented that the use of corticosteroid eye drops lead to the elevation of intraocular pressure (IOP) due to therapeutic use of glucocorticoids is called steroid-induced ocular hypertension (SIOH); this can lead to steroid-induced glaucoma (SIG) and increase risk of cataracts (Fini et al, 2017). There have been a few reports of ocular complications for topical corticosteroids, either applied around the eyes and eyelids or inadvertent contamination by rubbing eyes with unwashed hands, after topical steroid application (Berth-Jones, 2016). Prescribers should reduce this risk by emphasising good handwashing after topical corticosteroid application and wearing cotton gloves at night when treating hand eczema. The lowest potency of topical corticosteroid should be used on the eyelids for short treatment bursts only, with consideration to using topical calcineurin inhibitors as longer-term and maintenance treatment.
Topical immumodulators
Tacrolimus and pimecrolimus (both with similar actions but slightly different indications) are members of a new class of topical immunomodulators and belong to the class of immunosuppressant drugs known as calcineurin inhibitors. They work mainly by reducing inflammation through the suppression of T-lymphocyte responses, a different mechanism of action to topical corticosteroids (NICE, 2004). Topical immodulators are recommended by NICE as second line treatments for moderate to severe atopic eczema, for patients aged 2 years and upwards, where topical steroids have not gained eczema control or if there is a risk of side effects (NICE, 2004). Topical immumodualtors can be prescribed in primary care (where the prescriber has experience in managing eczema) and do not need to be initiated by a consultant dermatologist (PCDS, 2019; NICE, 2004).
Benefits
Topical immunomodulators have been in clinical practice for nearly 20 years. These targeted topical treatments have many benefits for patients with eczema. Tacrolimus and pimcrolimus both have positive effects on epidermal integrity, unlike steroids, there is no risk of skin atrophy (Papier and Strowd, 2018). Tacrolimus studies have shown benefits in improved skin barrier function, skin hydration, and skin thickness in patients with increased collagen synthesis, reversal of skin atrophy from topical corticosteroids (Kyllönen et al, 2004). Topical immunomodulators can be used in all body areas but are particularly useful for sensitive areas (including face (eyelids), neck, and flexures), which may be more susceptible to the adverse effects of topical corticosteroids.
Topical immodulators are applied thinly to areas of eczema, up to twice a day for six weeks and then can be continued twice a week, as long term maintenance therapy, to prevent and lessen flares. There are two formulations for tacrolimus (Protopic ointment), 0.1% is stronger and 0.03% is weaker). Adults and young people over the age of 16 years commencing on 0.1%, reducing to 0.03% if maintenance treatment is continued. Protopic 0.03% ointment is licensed from two years old, twice a day from the start of treatment for 3 weeks and then reduce to once a day until the atopic eczema is clear (Martins et al, 2015). There should be a two-hour gap between applying tacrolimus and emollient. Pimcrolimus (Elidel cream) is weaker than tacrolimus, licensed from two years old used for six weeks of initial treatment and then twice weekly maintenance. There is no direction for applying with emollient, so the usual 20-minute gap is recommended. If there is no improvement after 6 weeks, topical immunomodulators should be discontinued (Martins et al, 2015).
The efficacy of 0.1% tacrolimus has been demonstrated in several clinical trials, as being as effective as a potent topical corticosteroid, in adults and children, it was also better than low potency topical corticosteroid on the face and neck (Kyllönen et al, 2004). The lower dose 0.03% tacrolimus has been shown to be as effective as 0.1% in children only (Kyllönen et al, 2004). The risk-benefits of topical corticosteroids compared to topical immnomodulators have to also be weighed up with cost considerations, as tacrolimus and pimcrolimus are four times the cost (approximately £22.00/30 g) (Joint Formulary Committee, 2018).
Risks
The most common side effect with topical immodulators is a burning and stinging sensations when the cream or ointment is first applied to the skin. This will subside after a few applications and is rarely severe to withdraw treatment (Hengge et al, 2006). Other side effects include flushing on the face of a few users of tacrolimus ointment after alcohol (Kyllönen et al, 2004). There has been a suggestion of increased viral skin infections (molluscum, viral warts, herpes, eczema herpeticum), but Cochrane found no evidnce to support this risk (Kyllönen et al, 2004).
The main precaution for topical immunmodulators is to minimise exposure of the skin to sunlight and the use of ultraviolet (UV) light from sunbeds with UVB or UVA in combination with psoralens (PUVA) should be avoided during use of Protopic ointment (DermNetNZ, 2008). The reason for this advice is that in early preclinical studies potential for the development of skin cancers was seen with local immunosuppression. There is less concern for pimcrolimus than tacrolimus (DermNetNZ, 2008). Prescribers should advise on sun safety, including using a sunscreen with a minimum SPF30, and using protective clothing (Martins et al, 2015).
There can be concerns expressed by perscribers and patients regarding potential cancer risks with topical immunomodulators, as when tacrolimus and pimcrolimus were available to prescribe they carried a black box warning (due to the known increased risk of lymphoma and non lymphoma skin lesions for transplant patients prescribed oral tacrolimus). In 2015, a Cochrane stated that there was no evidence of an increased risk of malignancy with topical immunomodulators. Any cases of lymphoma reported were very low and the review concluded that it was possible these cases could have been cutaneous T lymphoma's misdiagnosed as atopic eczema (Martins et al, 2015). The review presented evidence that after topical application of tacrolimus, serum concentrations of the drug are usually low or undetectable, and rates of absorption decrease with improvement of the skin barrier integrity (Martins et al, 2015).
Antimicrobials
Bacterial colonisation with staphylococcus aureus complicates eczema. If a patient is not responding to topical steroids and the skin is inflamed, sore and weepy (with yellow crusts), bacterial infection should be suspected (Gong et al, 2006). NICE (2018) recommends that if there are extensive areas of infected eczema, skin should be swabbed (however, kno routine swabbing of eczema that is not infected is not recommended) and an oral antibiotic prescribed. NICE (2018) recommends appropriate treatments as below):
- Flucloxacillin is the first-line choice
- Prescribe erythromycin if the person has an allergy to penicillin or if there is known resistance to flucloxacillin. If the person has previously been unable to tolerate erythromycin because of nausea or cramps, consider prescribing clarithromycin
- If the infection responds poorly to the first-line antibiotic, prescribe an alternative antibiotic, if necessary, according to the swab results, or seek specialist advice.
For localised infection, a topical antibiotic (fusidic acid)/corticosteroid combination cream be prescribed for a 14 day course (NICE, 2007; 2018). In addition to reduce risk of bacterial load and re-infection, a topical antiseptic preparation (for example chlorhexidine or triclosan) as an adjunct to standard treatment, which will be prescribed an emollient product with added antimicrobial for washing and moisturising (NICE 2007; 2018; PCDS, 2019).
Benefits
Antimicrobials have benefits for treating eczematous skin infected with staphylococcus aureus (however, routine swabbing of eczema that is not infected is not recommended). A recent study, conducted in children with atopic eczema under 8 years old has concluded that less severely infected eczema should not be treated with antibiotics as long as treatment with standard steroid creams is offered, but this may not apply to children with more severe signs of infection (Francis et al, 2016).
Risks
Flucloxacillin should not be prescribed to people with a true penicillin allergy or history of penicillin-associated hepatic dysfunction. It should be prescribed with caution for people with hypersensitivity to cephalosporins, hepatic and renal impairment. Common side effects of flucloxacillin include nausea, vomiting, skin rash, and diarrhoea; possible drug interactions include anticoagulants, methotrexate (NICE, 2018).
Bacterial resistance to staphylococcus aureus has been reported since 1995 to occur with the use of topical fusidic acid. As with all antibiotics, extended or recurrent use may increase the risk of developing antibiotic resistance. In addition recurrent use may increase the risk of developing contact sensitisation (Williamson et al, 2017). Prescribers will be aware of the need to prescribe all antibiotics appropriately; and the UK 20 year vision and 5-year national action plan for containing and controlling antimicrobial resistance by 2040 (Public Health England (PHE), 2019).
Antihistamines
Antihistamines are frequently prescribed in primary care to treat itch and sedating antihistamines to help with sleeplessness. A Cochrane study, published in 2018, reviewed whether H1 antihistamine tablets improve eczema symptoms in people who are already using creams or ointments for their eczema. The study reviewed non-sedating antihistamines cetirizine, fexofenadine and loratidine. The conclusion of the review was there was no consistent or convincing evidence that H1 anti-histamines are effective ‘add-on’ treatments for eczema (PHE, 2019). Sedating antihistamines, such as chlorphenamine (Piriton) should only be prescribed at night-time to promote sleep and help break the itch–scratch cycle, but should not be used on a long-term basis (PCDS, 2019).
Benefits
There is no benefit in prescribing non-sedating H1 antihistamines for eczema symptoms. There is some benefit in prescribing sedating antihistamines to aid sleep, mainly for children with atopic eczema (NICE, 2007; PCDS, 2019).
Risks
The anticholinergic properties of chlorphenamine may cause drowsiness, dizziness, blurred vision and psychomotor impairment, which can seriously hamper the patients' ability to drive and use machinery. Other side effects include headaches and fatique (Matterne et al, 2019).
Table 1. Risk reduction in topical eczema management
| Topical Therapy | Risks | Risk reduction |
|---|---|---|
| Emollients | FlammabilitySlipping hazardsContact irritant/allergic dermatitis |
Issue safety guidance Warn patient Assess for irritancy, change emollient/allow patient choice Do not prescribe or recommend aqueous cream |
| Topical corticosteroids | Skin atrophy |
Prescribe correct potent according to age, body site and vehicle Use once a day Use for short term treatment bursts and limited longer term ‘weekend therapy’ |
| Exacerbation of skin Infection | Assess for signs of skin infection and treat accordingly | |
| Steroid-induced glaucaoma | Caution in prescribing around eyelids – consider topical immunomodulatorsWarn patients to wash hands after application to body | |
| Topical immunomodulators | Burning and stinging on application | Warn patients to expect this for the first few days of treatment |
| Facial flushing | Reduce alcohol intake | |
| Photosensitivity | Advise on sun protection measures | |
| Antimicrobials | Hypersensitivity to penicillin | Careful history taking, prevent penicillin-allergic reaction |
| Topical antibiotic resistance | Prescribe for a 14-day course and do not overuse | |
| Antihistamines | Drowsiness and dizziness |
Only prescribe sedating antihistamines for night-time use Do not prescribe non-sedating antihistamines |
Conclusion
This article has explored the benefits and risks of topical treatments in eczema management. Following evidence-based guidelines is important reinforcing ongoing preventative treatment with emollients and step up/step down with the topical treatments for short flare treatment or longer-term maintenance treatment. Patient's with moderate or severe eczema may not be adequately controlled with topical therapies alone, or may require secondary care assessment, including allergy testing (for contact dermatitis) and consideration of additional therapies, including phototherapy, systemic immunosuppressant drugs and biologic therapies (NICE 2018; 2007; PCDS, 2019).