Penthrox (methoxyflurane) is a well-known drug, which belongs to the fluorinated hydrocarbon group of volatile anesthetic agents. It is indicated for the emergency relief of moderate-to-severe pain in conscious adult patients with trauma and associated pain. It was used during the 1960s and 70s as an anaesthetic agent in surgical theatres. Multiples papers from that time show that given in large quantities, it is related with nephro- and hepatotoxicity (Crandell et al, 1966; Elkington et al, 1968) so it was removed from the formulary. Since then, it has been used predominately in Australia and New Zealand in much smaller doses as an analgesic for moderate-to-severe pain. However, in 2015, it was re-licenced for use in the UK as an analgesic for moderate to severe pain.
Use
Penthrox is indicated for emergency relief of moderate-to-severe pain in conscious adult patients with trauma and associated pain (Galen Limited, 2019). This includes burns, fractures or dislocations, which makes it a good drug to use in the pre-hospital setting (Forrest et al, 2019).
Recommendations
Methoxyflurane has some hypnotic and relaxant effects, and it is especially helpful with dislocated extremities or reducing fractures. It is a patient controlled analgesic (PCA); therefore, the patient has control of the required dose. It is very difficult to overdose on methoxyflurane and for the drug to have any central nervous system (CNS) depressant effect by itself due to its low dosage recommendations.
Posology and method of administration
Penthrox is available as a self-administered inhaler. Every pack comes with a 3 ml dose of methoxyflurane that needs to be poured into the inhaler at a 45° angle while rotating the container (Figure 1), before the administration, to make sure the drug spreads through the container. Once prepared, the patient must hold it in any angle upright.
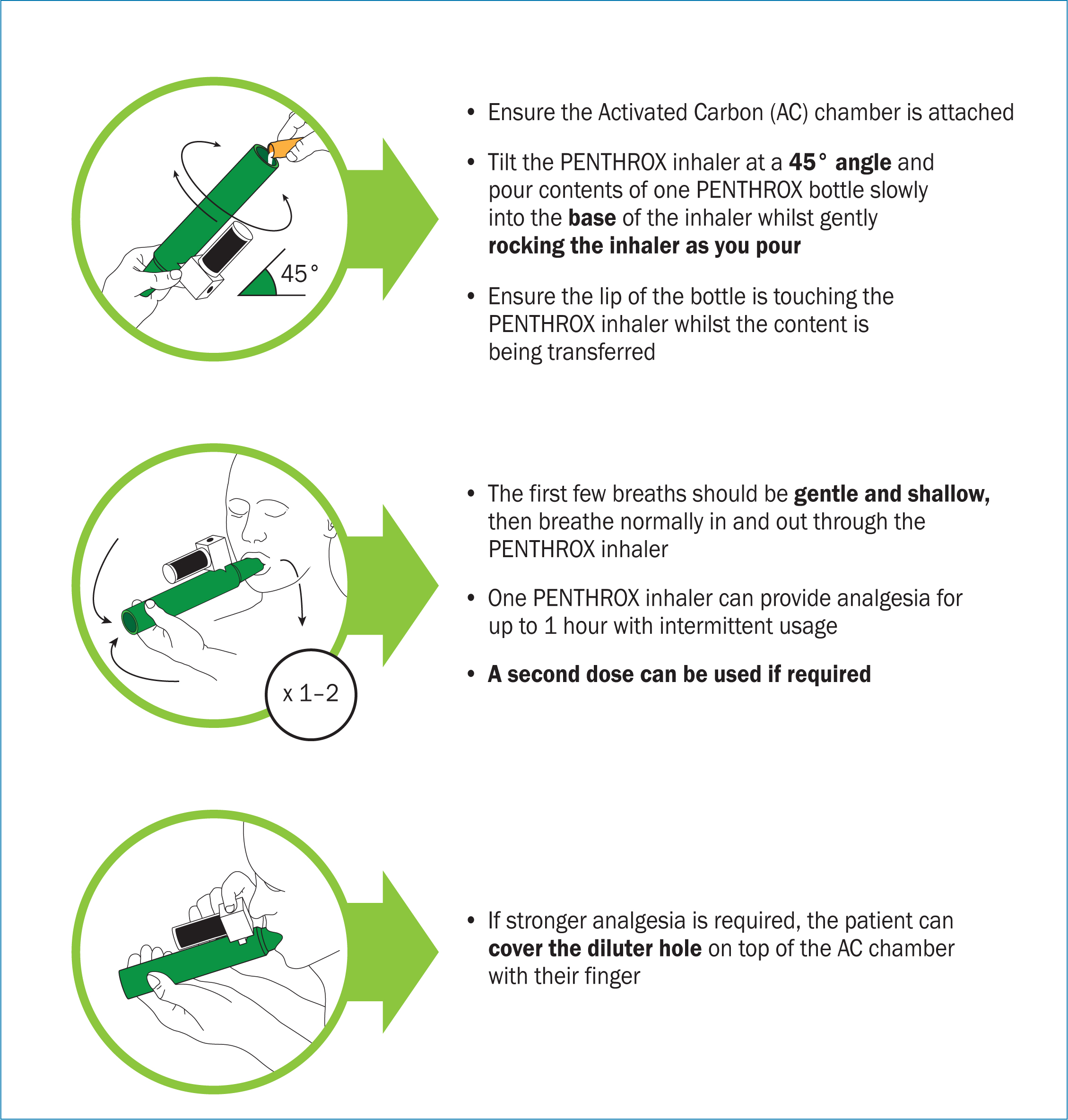
The drug is administered through inhalation; the patient breathes in from the mouthpiece and the analgesic effect occurs after 5–7 inhalations. Depending on the patient's level of pain, the dilutor hole in the activated carbon (AC) chamber can be covered to increase the concentration of inhaled methoxyflurane. It is important that the user of the inhaler also exhales using the device, so the residual exhaled methoxyflurane can be absorbed in to the AC chamber and accidental exposure is avoided.
If the administrator can smell the strong fruity odour of methoxyflurane, the medication may be administered incorrectly and the preparation and administration procedure should be reviewed. It would be of benefit for the patient to be made aware of the strong odour associated with methoxyflurane before administration.
Depending on usage, the inhaler can last from 30 minutes to 1 hour. After this, a new Penthrox inhaler will be needed.
The manufacturer recommends a maximum dose of 6 ml/day, or two complete inhalers. The maximum weekly dose should not exceed 15 ml. The lowest possible dose should be used to achieve pain relief.
Pharmacological properties
Pharmacodynamics
Methoxyflurane belongs to the fluorinated halogenated ether group of volatile anaesthetic agents. However, the mechanism by which methoxyflurane exerts its analgesic activity has not been fully elucidated (electronic Medicines Compendium (eMC), 2019). It provides an analgesic effect when inhaled at low concentrations and an anaesthetic effect at high concentrations. It exerts other effects on the body including some decrease in blood pressure, bradycardia and drowsiness (eMC, 2019).
Pharmacokinetic properties
Methoxyflurane is an inhaled analgesic that enters the lungs in the form of a vapour and is rapidly transported into the blood. Therefore, it has a rapid absorption and a quick mechanism of action. It is distributed by the blood and is highly lipophilic, it has great propensity to diffuse into fatty tissues where it forms a reservoir from which it is released slowly over days (Blair and Frampton, 2016).
Approximately 40% of the drug is exhaled unaltered or as carbon dioxide. The remaining 60% is metabolised in the liver by cytochrome P450 into organic fluorine, fluoride and oxalic acid are excreted in the urine. The metabolites can cause renal damage (eMC, 2019; Galen, 2019). Methoxyflurane is more susceptible to metabolism than other ethers due to its propensity to diffuse into fatty tissues. However, it is released slowly from its reservoir over many days; therefore, long-term effects have not been noted when Penthrox has been used in a controlled manner.
Contraindications, interactions and side effects
Contraindications
Volatile halogenated anaesthetics have the following contraindications (Joint Formulary Committee, 2019):
- Cardiovascular disease
- History of liver damage associated with use of methoxyflurane or other halogenated anaesthetics
- Impaired consciousness
- Respiratory depression
- Susceptibility to malignant hyperthermia.
Interactions
When used at the analgesic dosage of 3–6 ml for inhalation, methoxyflurane has not been associated with any drug interactions (Galen, 2019). However, the British National Formulary recommend (Joint Formulary Committee, 2019):
- Avoiding concurrent use with drugs with CNS depressant effects
- Avoiding use with antiepileptics (phenobarbital, primidone). Isoniazid and rifampicin will potentially increase the risk of nephrotoxicity
- Use with caution when patient is on medication that can cause hypotension (eg atenolol, avanafil).
Side effects
The following side effects have been noted with Penthrox (Galen, 2019):
- Cardiovascular effects: can lead to hypotension and bradycardia. It should not be used in unstable cardiovascular patients
- Respiratory effects: some adverse respiratory reactions were reported in clinical trials (eMC, 2018). It should not be used in evident respiratory depressed patients
- CNS: Penthrox is a CNS depressant and can produce symptoms such as sedation, euphoria or amnesia. Risk is increased when used with other CNS depressant
- Malignant hyperthermia: it can trigger malignant hyperthermia in genetically pre-disposed patients. Avoid in patients with a past medical history or a family history of malignant hyperthermia or severe adverse reactions.
Reproductive, genotoxicity and carciogenicity
Methoxyflurane is not considered mutagenic. However, there is not enough evidence to support that this drug has any reproductive, genotoxicity or carcinogenicity properties. This is the reason why the manufacturer recommends to use with caution.
Regarding pregnancy, methoxyflurane is known to cross the placenta. However, there is no evidence of toxicity to the fetus or teratogenicity.
Methoxyflurane was observed to show delayed fetal development following repeated doses for 9 days in rats (Lim et al, 2016).
Pros and cons
Pros
- Rapid effect
- Patient controlled analgesia allows adequate level of pain relief
- Easy route of administration
- Quick to wean off the effects
- Hypnotic and relaxant effect useful in trauma
Cons
- Contraindicated in respiratory depressed patients
- Difficult to use with adjuvants
- Requires a fully conscious and cooperative patient.
Storage precautions
Penthrox does not require temperature-controlled storage. It should be stored in a locked cupboard and never left unsupervised.
Comparison with other analgesics
There have been several studies published comparing methoxyflurane with other analgesics, such as nitrous oxide (Porter et al, 2017), intramuscular (IM), tramadol (Blair and Frampton, 2016), and intranasal (IN) fentanyl (Johnston et al, 2011).
Placebo
Coffey et al (2014) found that methoxyflurane reduced pain severity significantly more than a placebo with every test. They concluded that methoxyflurane is an efficacious, safe and fast-acting analgesic.
Intramuscular tramadol
According to Lim et al (2016), inhaled methoxyflurane relieved pain more rapidly than 100 mg of tramadol IM, and at 5 minutes the pain score was significantly lower. However, pain scores were not significantly different at 15 and 60 minutes. The patient's satisfaction was significantly higher with methoxyflurane than tramadol IM.
Intranasal fentanyl
Johnston et al (2010) carried out a retrospective, observational study between methoxyflurane and fentanyl IN. They found that methoxyflurane had a quicker onset of analgesia and both had similar analgesic power. Fentanyl lasted longer and achieved superior pain relief at hospital, especially for presumed cardiac pain in older, female patients.
Nitrous oxide
Porter's (2017) systematic review, did not find a significant difference between nitrous oxide and methoxyflurane. They are both suitable options for the pain treatment of trauma patients. However, penthrox is lighter, more portable, and has less contraindications. International studies directly comparing the use of both drugs are ongoing and will develop the evidence-base.
Conclusions
Methoxyflurane is a very useful, easy to administer and relatively safe drug for the initial treatment of trauma-related pain in a conscious patient. Like any other anaesthetic drugs, it must be administered under medical supervision due to its CNS depressant effects and its potential for hypotension. If this drug becomes more widely used, each Trust will have to declare an appropriate supervisor for its use among patient groups.
Key Points
- Methoxyflurane is a safe, self-administered inhalatory analgesic drug with a rapid onset
- It is indicated for emergency relief of moderate-to-severe pain in conscious adults with trauma and associated pain
- It has great propensity to diffuse into fatty tissues where it forms a reservoir from which it is released slowly over days
- It is not considered to have mutagenic, toxicity to the fetus or teratogenic properties
CPD reflective questions
- Can Penthrox make a difference to the analgesic approach in your clinical area? What barriers, if any, do you see for the introduction of this drug in your clinical setting?
- Take some time to compare Penthrox to the alternatives used in your workplace

